Fragmentation of Rapid Eye Movement and Nonrapid Eye Movement Sleep without Total Sleep Loss Impairs Hippocampus-Dependent Fear Memory Consolidation
- PMID: 27568801
- PMCID: PMC5070756
- DOI: 10.5665/sleep.6236
Fragmentation of Rapid Eye Movement and Nonrapid Eye Movement Sleep without Total Sleep Loss Impairs Hippocampus-Dependent Fear Memory Consolidation
Abstract
Study objectives: Sleep is important for consolidation of hippocampus-dependent memories. It is hypothesized that the temporal sequence of nonrapid eye movement (NREM) sleep and rapid eye movement (REM) sleep is critical for the weakening of nonadaptive memories and the subsequent transfer of memories temporarily stored in the hippocampus to more permanent memories in the neocortex. A great body of evidence supporting this hypothesis relies on behavioral, pharmacological, neural, and/or genetic manipulations that induce sleep deprivation or stage-specific sleep deprivation.
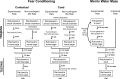
Methods: We exploit an experimental model of circadian desynchrony in which intact animals are not deprived of any sleep stage but show fragmentation of REM and NREM sleep within nonfragmented sleep bouts. We test the hypothesis that the shortening of NREM and REM sleep durations post-training will impair memory consolidation irrespective of total sleep duration.
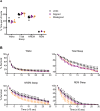
Results: When circadian-desynchronized animals are trained in a hippocampus-dependent contextual fear-conditioning task they show normal short-term memory but impaired long-term memory consolidation. This impairment in memory consolidation is positively associated with the post-training fragmentation of REM and NREM sleep but is not significantly associated with the fragmentation of total sleep or the total amount of delta activity. We also show that the sleep stage fragmentation resulting from circadian desynchrony has no effect on hippocampus-dependent spatial memory and no effect on hippocampus-independent cued fear-conditioning memory.
Conclusions: Our findings in an intact animal model, in which sleep deprivation is not a confounding factor, support the hypothesis that the stereotypic sequence and duration of sleep stages play a specific role in long-term hippocampus-dependent fear memory consolidation.
Keywords: NREM sleep fragmentation; REM sleep fragmentation; circadian desynchrony; memory consolidation.
© 2016 Associated Professional Sleep Societies, LLC.
Figures





Similar articles
-
Abnormal Locus Coeruleus Sleep Activity Alters Sleep Signatures of Memory Consolidation and Impairs Place Cell Stability and Spatial Memory.Curr Biol. 2018 Nov 19;28(22):3599-3609.e4. doi: 10.1016/j.cub.2018.09.054. Epub 2018 Nov 1. Curr Biol. 2018. PMID: 30393040 Free PMC article.
-
Sleep-dependent consolidation of face recognition and its relationship to REM sleep duration, REM density and Stage 2 sleep spindles.J Sleep Res. 2017 Jun;26(3):318-321. doi: 10.1111/jsr.12520. Epub 2017 Mar 31. J Sleep Res. 2017. PMID: 28370532 Clinical Trial.
-
Post-learning paradoxical sleep deprivation impairs reorganization of limbic and cortical networks associated with consolidation of remote contextual fear memory in mice.Sleep. 2018 Dec 1;41(12). doi: 10.1093/sleep/zsy188. Sleep. 2018. PMID: 30285241
-
The role of rapid eye movement sleep for amygdala-related memory processing.Neurobiol Learn Mem. 2015 Jul;122:110-21. doi: 10.1016/j.nlm.2015.01.008. Epub 2015 Jan 28. Neurobiol Learn Mem. 2015. PMID: 25638277 Review.
-
Consolidative mechanisms of emotional processing in REM sleep and PTSD.Sleep Med Rev. 2018 Oct;41:173-184. doi: 10.1016/j.smrv.2018.03.001. Epub 2018 Mar 15. Sleep Med Rev. 2018. PMID: 29628334 Review.
Cited by
-
GBA-AAV mitigates sleep disruptions and motor deficits in mice with REM sleep behavior disorder.NPJ Parkinsons Dis. 2024 Aug 2;10(1):142. doi: 10.1038/s41531-024-00756-5. NPJ Parkinsons Dis. 2024. PMID: 39095359 Free PMC article.
-
Cortical activation and functional connectivity in patients with chronic insomnia based on working memory tasks: a multi-channel near-infrared spectroscopy study.BMC Psychiatry. 2025 Aug 14;25(1):788. doi: 10.1186/s12888-025-07177-5. BMC Psychiatry. 2025. PMID: 40813976 Free PMC article.
-
The Dorsal Medial Habenula Minimally Impacts Circadian Regulation of Locomotor Activity and Sleep.J Biol Rhythms. 2017 Oct;32(5):444-455. doi: 10.1177/0748730417730169. Epub 2017 Sep 27. J Biol Rhythms. 2017. PMID: 28954569 Free PMC article.
-
Circadian regulation of sleep in a pre-clinical model of Dravet syndrome: dynamics of sleep stage and siesta re-entrainment.Sleep. 2019 Dec 24;42(12):zsz173. doi: 10.1093/sleep/zsz173. Sleep. 2019. PMID: 31346614 Free PMC article.
-
Vocabulary learning benefits from REM after slow-wave sleep.Neurobiol Learn Mem. 2017 Oct;144:102-113. doi: 10.1016/j.nlm.2017.07.001. Epub 2017 Jul 8. Neurobiol Learn Mem. 2017. PMID: 28697944 Free PMC article.
References
-
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. - PubMed
-
- Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci. 2011;15:343–51. - PubMed
-
- de la Iglesia HO, Cambras T, Schwartz WJ, Diez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004;14:796–800. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

