Wild-Type Transthyretin Cardiac Amyloidosis: Novel Insights From Advanced Imaging
- PMID: 27568874
- PMCID: PMC5004088
- DOI: 10.1016/j.cjca.2016.05.008
Wild-Type Transthyretin Cardiac Amyloidosis: Novel Insights From Advanced Imaging
Abstract
Amyloidosis is caused by extracellular deposition of abnormal protein fibrils, resulting in destruction of tissue architecture and impairment of organ function. The most common forms of systemic amyloidosis are light-chain and transthyretin-related (ATTR). ATTR can result from an autosomal dominant hereditary transmission of mutated genes in the transthyretin or from a wild-type form of disease (ATTRwt), previously known as senile cardiac amyloidosis. With the aging of the worldwide population, ATTRwt will emerge as the most common type of cardiac amyloidosis that clinicians encounter. Diagnosis of systemic amyloidosis is often delayed, either because of the false assumption that it is a rare disease, or because of misdiagnosis as a result of mistaking it with other conditions. Clinicians must integrate clinical clues from history, physical examination, and common diagnostic tests to raise suspicion for ATTRwt. The historical gold standard for diagnosis of cardiac amyloid is endomyocardial biopsy analysis with pathological distinction of precursor protein type, but this method often results in delayed diagnosis because of the limited availability of expertise to perform and interpret the endomyocardial biopsy specimen. Emerging noninvasive imaging modalities provide easier, accurate screening for ATTRwt. These modalities include advanced echocardiography, using strain imaging and the myocardial contraction fraction; nuclear scintigraphy, which can differentiate between ATTR and light-chain cardiac amyloid; and cardiac magnetic resonance imaging, using extracellular volume measurement, late gadolinium enhancement, and distinct T1 mapping. These novel approaches reveal insights into the prevalence, clinical course, morphological effects, and prognosis of ATTRwt.
Copyright © 2016 Canadian Cardiovascular Society. Published by Elsevier Inc. All rights reserved.
Figures
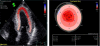

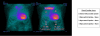

References
-
- Rapezzi C, Lorenzini M, Longhi S, et al. Cardiac amyloidosis: the great pretender. Heart Fail Rev. 2015;20:117–124. - PubMed
-
- Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. - PubMed
-
- Mohty D, Damy T, Cosnay P, et al. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis. 2013;106:528–540. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

