Development of Improved Vaccine Adjuvants Based on the Saponin Natural Product QS-21 through Chemical Synthesis
- PMID: 27568877
- PMCID: PMC5032057
- DOI: 10.1021/acs.accounts.6b00242
Development of Improved Vaccine Adjuvants Based on the Saponin Natural Product QS-21 through Chemical Synthesis
Abstract
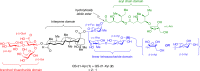
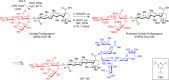
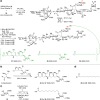
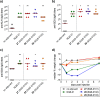
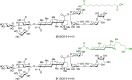
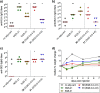
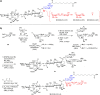
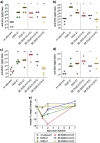
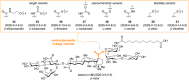
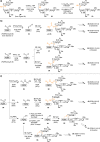
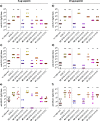
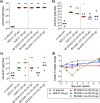
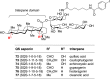
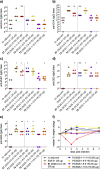
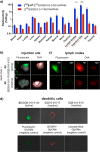
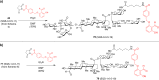
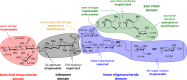
Vaccines based on molecular subunit antigens are increasingly being investigated due to their improved safety and more precise targeting compared to classical whole-pathogen vaccines. However, subunit vaccines are inherently less immunogenic; thus, coadministration of an adjuvant to increase the immunogenicity of the antigen is often necessary to elicit a potent immune response. QS-21, an immunostimulatory saponin natural product, has been used as an adjuvant in conjunction with various vaccines in numerous clinical trials, but suffers from several inherent liabilities, including scarcity, chemical instability, and dose-limiting toxicity. Moreover, little is known about its mechanism of action. Over a decade-long effort, beginning at the University of Illinois at Urbana-Champaign and continuing at the Memorial Sloan Kettering Cancer Center (MSKCC), the group of Prof. David Y. Gin accomplished the total synthesis of QS-21 and developed a practical semisynthetic approach to novel variants that overcome the liabilities of the natural product. First, semisynthetic QS-21 variants were designed with stable amide linkages in the acyl chain domain that exhibited comparable in vivo adjuvant activity and lower toxicity than the natural product. Further modifications in the acyl chain domain and truncation of the linear tetrasaccharide domain led to identification of a trisaccharide variant with a simple carboxylic acid side chain that retained potent adjuvant activity, albeit with reemergence of toxicity. Conversely, an acyl chain analogue terminating in a free amine was inactive but enabled chemoselective functionalization with radiolabeled and fluorescent tags, yielding adjuvant-active saponin probes that, unlike inactive congeners, accumulated in the lymph nodes in vaccinated mice and internalized into dendritic cells. Subtle variations in length, stereochemistry, and conformational flexibility around the central glycosidic linkage provided QS-21 variants with adjuvant activities that correlated with specific conformations found in molecular dynamics simulations. Notably, deletion of the entire branched trisaccharide domain afforded potent, truncated saponin variants with negligible toxicity and improved synthetic access, facilitating subsequent investigation of the triterpene core. The triterpene C4-aldehyde substituent, previously proposed to be important for QS-21 adjuvant activity, proved to be dispensable in these truncated saponin variants, while the presence of the C16 hydroxyl group enhanced activity. Novel adjuvant conjugates incorporating the small-molecule immunopotentiator tucaresol at the acyl chain terminus afforded adjuvant-active variants but without significant synergistic enhancement of activity. Finally, a new divergent synthetic approach was developed to provide versatile and streamlined access to additional linear oligosaccharide domain variants with modified sugars and regiochemistries, opening the door to the rapid generation of diverse, synthetically accessible analogues. In this Account, we summarize these multidisciplinary studies at the interface of chemistry, immunology, and medicine, which have provided critical information on the structure-activity relationships (SAR) of this Quillaja saponin class; access to novel, potent, nontoxic adjuvants for use in subunit vaccines; and a powerful platform for investigations into the mechanisms of saponin immunopotentiation.
Conflict of interest statement
The authors declare the following competing financial interests: A.F.-T., D.S.T., and D.Y.G. are coinventors on patents and patent applications based on this work. D.Y.G. was a cofounder of, and his estate holds financial interests in, Adjuvance Technologies, Inc., which has licensed certain technologies described herein.
Figures







Similar articles
-
Semisynthesis of Analogues of the Saponin Immunoadjuvant QS-21.Methods Mol Biol. 2017;1494:45-71. doi: 10.1007/978-1-4939-6445-1_4. Methods Mol Biol. 2017. PMID: 27718185 Free PMC article.
-
Development of a minimal saponin vaccine adjuvant based on QS-21.Nat Chem. 2014 Jul;6(7):635-43. doi: 10.1038/nchem.1963. Epub 2014 Jun 1. Nat Chem. 2014. PMID: 24950335 Free PMC article.
-
Design, synthesis, and immunologic evaluation of vaccine adjuvant conjugates based on QS-21 and tucaresol.Bioorg Med Chem. 2014 Nov 1;22(21):5917-23. doi: 10.1016/j.bmc.2014.09.016. Epub 2014 Sep 17. Bioorg Med Chem. 2014. PMID: 25284254 Free PMC article.
-
Advances in saponin-based adjuvants.Vaccine. 2009 Mar 13;27(12):1787-96. doi: 10.1016/j.vaccine.2009.01.091. Epub 2009 Feb 7. Vaccine. 2009. PMID: 19208455 Review.
-
Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer.Expert Rev Vaccines. 2011 Apr;10(4):463-70. doi: 10.1586/erv.11.18. Expert Rev Vaccines. 2011. PMID: 21506644 Free PMC article. Review.
Cited by
-
A Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development.ACS Cent Sci. 2021 Apr 28;7(4):512-533. doi: 10.1021/acscentsci.1c00120. Epub 2021 Mar 29. ACS Cent Sci. 2021. PMID: 34056083 Free PMC article.
-
Pressurized Hot Water Extraction as Green Technology for Natural Products as Key Technology with Regard to Hydrodistillation and Solid-Liquid Extraction.ACS Omega. 2024 Jul 10;9(29):31998-32010. doi: 10.1021/acsomega.4c03771. eCollection 2024 Jul 23. ACS Omega. 2024. PMID: 39072122 Free PMC article.
-
cGAMP/Saponin Adjuvant Combination Improves Protective Response to Influenza Vaccination by Microneedle Patch in an Aged Mouse Model.Front Immunol. 2021 Feb 2;11:583251. doi: 10.3389/fimmu.2020.583251. eCollection 2020. Front Immunol. 2021. PMID: 33603732 Free PMC article.
-
Development of a New Vaccine Adjuvant System Based on the Combination of the Synthetic TLR4 Agonist FP20 and a Synthetic QS-21 Variant.J Med Chem. 2024 Dec 26;67(24):22254-22262. doi: 10.1021/acs.jmedchem.4c02392. Epub 2024 Dec 8. J Med Chem. 2024. PMID: 39645607 Free PMC article.
-
Synthesis and Evaluation of a QS-17/18-Based Vaccine Adjuvant.J Med Chem. 2019 Feb 14;62(3):1669-1676. doi: 10.1021/acs.jmedchem.8b01997. Epub 2019 Jan 18. J Med Chem. 2019. PMID: 30656932 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

