Pharmacokinetics of a Novel Zolpidem Nasal Spray for Rapid Management of Insomnia: First Trial in Humans
- PMID: 27568900
- PMCID: PMC5078699
- DOI: 10.5664/jcsm.6264
Pharmacokinetics of a Novel Zolpidem Nasal Spray for Rapid Management of Insomnia: First Trial in Humans
Abstract
Study objectives: The present single-dose, parallel-group, randomized, double-blind, placebo-controlled study is to evaluate the pharmacokinetics, tolerability and safety of zolpidem tartrate nasal spray (ZNS) as compared to placebo in healthy subjects.
Methods: Thirty-six healthy subjects participated in this study, with 19 male and 17 female subjects in 3 cohorts (12 subjects per cohort), who were randomly assigned to receive either an intranasal dose of ZNS 1.75 mg, 3.5 mg, 5.0 mg (n = 10 per dose), or an intranasal placebo (n = 2). Multiple venous blood samples were collected for pharmacokinetic analyses.
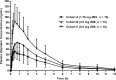
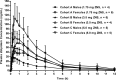
Results: Plasma zolpidem concentrations rapidly increased after intranasal ZNS 1.75, 3.5, and 5.0 mg with mean Tmax of 0.42, 0.76 and 0.50 h, respectively, followed by rapid decreases at all three doses. Cmax, AUC0-t, and AUC0-∞ were found to increase in a dose-proportional manner. Female subjects had generally higher AUC0-t, AUC0-∞, and lower weight-normalized clearance rate (CL/F) than male subjects. In this study, ZNS was safe and well tolerated over the evaluated dose range. There were no serious adverse events.
Conclusions: Zolpidem was rapidly absorbed and eliminated after intranasal administration of ZNS. Dose proportionality was found at the doses ranged from 1.75 mg to 5.0 mg. Intranasal exposure of zolpidem was generally higher in female subjects than that in male subjects. It could be concluded that ZNS is safe and well tolerated over the evaluated range of intranasal doses.
Keywords: drug metabolism; hypnotics; insomnia; middle-of-the-night awakening; pharmacokinetics.
© 2016 American Academy of Sleep Medicine
Figures
Similar articles
-
Pharmacokinetic effects of simultaneous administration of single-dose gabapentin 500 mg and zolpidem tartrate 10 mg in healthy volunteers: a randomized, open-label, crossover trial.Drugs R D. 2015 Mar;15(1):71-7. doi: 10.1007/s40268-014-0079-z. Drugs R D. 2015. PMID: 25567214 Free PMC article. Clinical Trial.
-
Low-dose sublingual zolpidem tartrate is associated with dose-related improvement in sleep onset and duration in insomnia characterized by middle-of-the-night (MOTN) awakenings.Sleep. 2008 Sep;31(9):1277-84. Sleep. 2008. PMID: 18788653 Free PMC article. Clinical Trial.
-
Safety, Tolerability, and Pharmacokinetic Study of 101BHG-D01 Nasal Spray, a Novel Long-Acting and Selective Cholinergic M Receptor Antagonist, in Healthy Chinese Volunteers: A Randomized, Double-Blind, Placebo-Controlled, Single-Dose Escalation, First-In-Human Study.Eur J Drug Metab Pharmacokinet. 2022 Jul;47(4):509-521. doi: 10.1007/s13318-022-00769-6. Epub 2022 Apr 16. Eur J Drug Metab Pharmacokinet. 2022. PMID: 35429285 Clinical Trial.
-
Zolpidem's use for insomnia.Asian J Psychiatr. 2017 Feb;25:79-90. doi: 10.1016/j.ajp.2016.10.006. Epub 2016 Oct 12. Asian J Psychiatr. 2017. PMID: 28262178 Review.
-
Sublingual zolpidem (Edluar™; Sublinox™).CNS Drugs. 2012 Nov;26(11):1003-10. doi: 10.1007/s40263-012-0009-y. CNS Drugs. 2012. PMID: 23034583 Review.
Cited by
-
Zolpidem Stimulant Effect and Dependence: A Case Report on Intranasal Use.Psychopharmacol Bull. 2024 Apr 4;54(2):34-38. Psychopharmacol Bull. 2024. PMID: 38601832 Free PMC article.
References
-
- Doghramji K. The epidemiology and diagnosis of insomnia. Am J Manag Care. 2006;12:S214–20. - PubMed
-
- Leger D, Poursain B. An international survey of insomnia: under-recognition and under-treatment of a polysymptomatic condition. Curr Med Res Opin. 2005;21:1785–92. - PubMed
-
- Roth T, Drake C. Evolution of insomnia: current status and future direction. Sleep Med. 2004;5:S23–30. - PubMed
-
- Kao CC, Huang CJ, Wang MY, Tsai PS. Insomnia: prevalence and its impact on excessive daytime sleepiness and psychological well-being in the adult Taiwanese population. Qual Life Res. 2008;17:1073–80. - PubMed
-
- Wong WS, Fielding R. Prevalence of insomnia among Chinese adults in Hong Kong: a population-based study. J Sleep Res. 2011;20:117–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

