The Yersinia Virulence Factor YopM Hijacks Host Kinases to Inhibit Type III Effector-Triggered Activation of the Pyrin Inflammasome
- PMID: 27569559
- PMCID: PMC5025386
- DOI: 10.1016/j.chom.2016.07.018
The Yersinia Virulence Factor YopM Hijacks Host Kinases to Inhibit Type III Effector-Triggered Activation of the Pyrin Inflammasome
Abstract
Pathogenic Yersinia, including Y. pestis, the agent of plague in humans, and Y. pseudotuberculosis, the related enteric pathogen, deliver virulence effectors into host cells via a prototypical type III secretion system to promote pathogenesis. These effectors, termed Yersinia outer proteins (Yops), modulate multiple host signaling responses. Studies in Y. pestis and Y. pseudotuberculosis have shown that YopM suppresses infection-induced inflammasome activation; however, the underlying molecular mechanism is largely unknown. Here we show that YopM specifically restricts the pyrin inflammasome, which is triggered by the RhoA-inactivating enzymatic activities of YopE and YopT, in Y. pseudotuberculosis-infected macrophages. The attenuation of a yopM mutant is fully reversed in pyrin knockout mice, demonstrating that YopM inhibits pyrin to promote virulence. Mechanistically, YopM recruits and activates the host kinases PRK1 and PRK2 to negatively regulate pyrin by phosphorylation. These results show how a virulence factor can hijack host kinases to inhibit effector-triggered pyrin inflammasome activation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
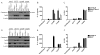
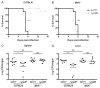
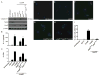



Comment in
-
The Type III Secretion System Cleans up Its Act(in).Cell Host Microbe. 2016 Sep 14;20(3):275-276. doi: 10.1016/j.chom.2016.08.012. Cell Host Microbe. 2016. PMID: 27631695
References
-
- AKTORIES K. Bacterial protein toxins that modify host regulatory GTPases. Nat Rev Microbiol. 2011;9:487–98. - PubMed
-
- AKULA MK, SHI M, JIANG Z, FOSTER CE, MIAO D, LI AS, ZHANG X, GAVIN RM, FORDE SD, GERMAIN G, CARPENTER S, ROSADINI CV, GRITSMAN K, CHAE JJ, HAMPTON R, SILVERMAN N, GRAVALLESE EM, KAGAN JC, FITZGERALD KA, KASTNER DL, GOLENBOCK DT, BERGO MO, WANG D. Control of the innate immune response by the mevalonate pathway. Nat Immunol 2016 - PMC - PubMed
-
- AUBERT DF, XU H, YANG J, SHI X, GAO W, LI L, BISARO F, CHEN S, VALVANO MA, SHAO F. A Burkholderia Type VI Effector Deamidates Rho GTPases to Activate the Pyrin Inflammasome and Trigger Inflammation. Cell Host Microbe 2016 - PubMed
-
- BERNEKING L, SCHNAPP M, RUMM A, TRASAK C, RUCKDESCHEL K, ALAWI M, GRUNDHOFF A, KIKHNEY AG, KOCH-NOLTE F, BUCK F, PERBANDT M, BETZEL C, SVERGUN DI, HENTSCHKE M, AEPFELBACHER M. Immunosuppressive Yersinia Effector YopM Binds DEAD Box Helicase DDX3 to Control Ribosomal S6 Kinase in the Nucleus of Host Cells. PLoS Pathog. 2016;12:e1005660. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

