Cold-inducible RNA-binding protein activates splenic T cells during sepsis in a TLR4-dependent manner
- PMID: 27569563
- PMCID: PMC5827170
- DOI: 10.1038/cmi.2016.43
Cold-inducible RNA-binding protein activates splenic T cells during sepsis in a TLR4-dependent manner
Abstract
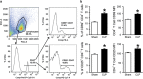
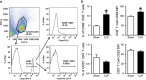
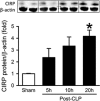
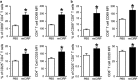
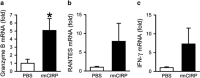
Cold-inducible RNA-binding protein (CIRP) is a novel inflammatory mediator that stimulates the release of proinflammatory cytokines from macrophages in sepsis. Given the immune dysregulation that characterizes sepsis, the effect of CIRP on other immune cells is an area of increasing interest that has not yet been studied. In the present study, we hypothesized that extracellular CIRP promotes activation of T lymphocytes in the spleen during sepsis. We observed that mice subjected to sepsis by cecal ligation and puncture showed significantly higher expression of the early activation markers CD69 and CD25 at 20 h on CD4+ splenic T cells, and significantly higher CD69 expression on CD8+ splenic T cells compared with sham-operated controls. Furthermore, at 20 h after receiving intravenous injection of recombinant murine CIRP (rmCIRP, 5 mg/kg body weight (BW)) or PBS (vehicle), those mice receiving rmCIRP showed significantly increased expression of CD69 and CD25 on both CD4+ and CD8+ splenic T cells. This effect, however, was not seen in TLR4-deficient mice after rmCIRP injection. In addition, treatment with CIRP predisposed CD4+ T cells to a Th1 hyperinflammatory response profile, and influenced CD8+ T cells toward a cytotoxic profile. Taken together, our findings indicate that CIRP is a proinflammatory mediator that plays an important role in T-cell dysregulation during sepsis in a TLR4-dependent manner.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369: 2063. - PubMed
-
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193: 259–272. - PubMed
-
- Scumpia PO, Delano MJ, Kelly KM, O'Malley KA, Efron PA, McAuliffe PF et al. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006; 177: 7943–7949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

