Management of gastric and duodenal neuroendocrine tumors
- PMID: 27570419
- PMCID: PMC4974581
- DOI: 10.3748/wjg.v22.i30.6817
Management of gastric and duodenal neuroendocrine tumors
Abstract
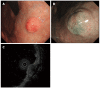
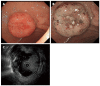
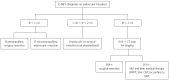
Gastrointestinal neuroendocrine tumors (GI-NETs) are rare neoplasms, like all NETs. However, the incidence of GI-NETS has been increasing in recent years. Gastric NETs (G-NETs) and duodenal NETs (D-NETs) are the common types of upper GI-NETs based on tumor location. G-NETs are classified into three distinct subgroups: type I, II, and III. Type I G-NETs, which are the most common subtype (70%-80% of all G-NETs), are associated with chronic atrophic gastritis, including autoimmune gastritis and Helicobacter pylori associated atrophic gastritis. Type II G-NETs (5%-6%) are associated with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome (MEN1-ZES). Both type I and II G-NETs are related to hypergastrinemia, are small in size, occur in multiple numbers, and are generally benign. In contrast, type III G-NETs (10%-15%) are not associated with hypergastrinemia, are large-sized single tumors, and are usually malignant. Therefore, surgical resection and chemotherapy are generally necessary for type III G-NETs, while endoscopic resection and follow-up, which are acceptable for the treatment of most type I and II G-NETs, are only acceptable for small and well differentiated type III G-NETs. D-NETs include gastrinomas (50%-60%), somatostatin-producing tumors (15%), nonfunctional serotonin-containing tumors (20%), poorly differentiated neuroendocrine carcinomas (< 3%), and gangliocytic paragangliomas (< 2%). Most D-NETs are located in the first or second part of the duodenum, with 20% occurring in the periampullary region. Therapy for D-NETs is based on tumor size, location, histological grade, stage, and tumor type. While endoscopic resection may be considered for small nonfunctional D-NETs (G1) located in the higher papilla region, surgical resection is necessary for most other D-NETs. However, there is no consensus regarding the ideal treatment of D-NETs.
Keywords: Classification; Duodenal neuroendocrine tumors; Endoscopic submucosal dissection; Endoscopic treatment; Gastric neuroendocrine tumors.
Figures
Similar articles
-
Management of Other Gastric and Duodenal Neuroendocrine Tumors.Surg Oncol Clin N Am. 2020 Apr;29(2):253-266. doi: 10.1016/j.soc.2019.11.009. Surg Oncol Clin N Am. 2020. PMID: 32151359 Review.
-
[Gastric neuroendocrine tumors].Khirurgiia (Mosk). 2019;(12):111-120. doi: 10.17116/hirurgia2019121111. Khirurgiia (Mosk). 2019. PMID: 31825351 Russian.
-
[Management of patients with neuroendocrine tumors of the esophagus, stomach, and duodenum].Nihon Geka Gakkai Zasshi. 2008 May;109(3):147-51. Nihon Geka Gakkai Zasshi. 2008. PMID: 18536318 Review. Japanese.
-
Gastric and duodenal neuroendocrine tumours.Best Pract Res Clin Gastroenterol. 2012 Dec;26(6):719-35. doi: 10.1016/j.bpg.2013.01.002. Best Pract Res Clin Gastroenterol. 2012. PMID: 23582915 Review.
-
Last decade of advances in gastric neuroendocrine tumors: Innovations, challenges, and future directions.World J Clin Oncol. 2025 May 24;16(5):104577. doi: 10.5306/wjco.v16.i5.104577. World J Clin Oncol. 2025. PMID: 40503415 Free PMC article.
Cited by
-
Update understanding on diagnosis and histopathological examination of atrophic gastritis: A review.World J Gastrointest Oncol. 2024 Oct 15;16(10):4080-4091. doi: 10.4251/wjgo.v16.i10.4080. World J Gastrointest Oncol. 2024. PMID: 39473965 Free PMC article. Review.
-
Endoscopic features of gastric neuroendocrine tumors.DEN Open. 2025 Feb 26;5(1):e70088. doi: 10.1002/deo2.70088. eCollection 2025 Apr. DEN Open. 2025. PMID: 40017512 Free PMC article.
-
Diagnostic performance and clinical impact of 18F-AlF-NOTA-octreotide in a large cohort of patients with neuroendocrine neoplasms: A prospective single-center study.Theranostics. 2024 May 19;14(8):3213-3220. doi: 10.7150/thno.96762. eCollection 2024. Theranostics. 2024. PMID: 38855183 Free PMC article.
-
Exploring the neglected segment of the intestine: the duodenum and its pathologies.Pol J Radiol. 2020 May 8;85:e230-e244. doi: 10.5114/pjr.2020.95477. eCollection 2020. Pol J Radiol. 2020. PMID: 32612721 Free PMC article. Review.
-
Safety and efficacy of endoscopic resection for the treatment of duodenal subepithelial lesions.J Gastrointest Oncol. 2021 Apr;12(2):856-863. doi: 10.21037/jgo-20-301. J Gastrointest Oncol. 2021. PMID: 34012672 Free PMC article.
References
-
- Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18, vii. - PubMed
-
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. - PubMed
-
- Rindi G, Arnold R, Bosman FT, Capella C, Klimstra DS, Klöppel G, Komminoth P, Solcia E. Nomenctlature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of Tumours of the digestive system. Lyon: IARC; 2010. pp. 13–14.
-
- Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563–2569. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

