Gamma-secretase Inhibitor Prevents Proliferation and Migration of Ductus Arteriosus Smooth Muscle Cells through the Notch3-HES1/2/5 Pathway
- PMID: 27570480
- PMCID: PMC4997050
- DOI: 10.7150/ijbs.16430
Gamma-secretase Inhibitor Prevents Proliferation and Migration of Ductus Arteriosus Smooth Muscle Cells through the Notch3-HES1/2/5 Pathway
Abstract
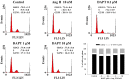
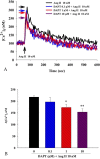
Patent ductus arteriosus (PDA) can cause morbidity and mortality in neonates. Vascular remodeling, characterized by proliferation and migration of smooth muscle cells (SMCs), is an essential process for postnatal DA closure. Notch signaling is an important mediator of vascular remodelling but its role in DA is unkonwn. We investigated the effects and underlying mechanisms of γ-secretase inhibitor DAPT, a Notch signaling inhibitor on angiotensin II (Ang II)-induced proliferation and migration of DASMCs. Proliferation and migration of DASMCs cultured from neonatal Wistar rats were induced by Ang II, with or without DAPT pre-treatment. In addition, potential underlying mechanisms including cell cycle progression, Ca(2+) influx, reactive oxygen species (ROS) production, signal transduction of MAPK and Akt, and Notch receptor with its target gene pathway were examined. We found that DAPT inhibited Ang II-induced DASMCs proliferation and migration dose dependently. DAPT also arrested the cell cycle progression in the G0/G1-phase, and attenuated calcium overload and ROS production caused by Ang II. Moreover, DAPT inhibited nuclear translocation of Notch3 receptor intracellular domain, with decreased expression of its down-stream genes including HES1, HES2 and HES5. Finally, Ang II-activated ERK1/2, JNK and Akt were also counteracted by DAPT. In conclusion, DAPT inhibits Ang II-induced DASMCs proliferation and migration. These effects are potentially mediated by decreased calcium influx, reduced ROS production, and down-regulation of ERK1/2, JNK and Akt, through the Notch3-HES1/2/5 pathway. Therefore, Notch signaling has a role in DA remodeling and may provide a target pathway for therapeutic intervention of PDA.
Keywords: Ductus arteriosus; Notch signaling; remodeling.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures






Similar articles
-
B-type natriuretic peptide inhibits angiotensin II-induced proliferation and migration of pulmonary arterial smooth muscle cells.Pediatr Pulmonol. 2014 Aug;49(8):734-44. doi: 10.1002/ppul.22904. Epub 2013 Oct 25. Pediatr Pulmonol. 2014. PMID: 24167111
-
B-type natriuretic peptide prevents postnatal closure of ductus arteriosus by both vasodilation and anti-remodeling in neonatal rats.Clin Sci (Lond). 2018 Sep 18;132(18):2045-2058. doi: 10.1042/CS20180201. Print 2018 Sep 28. Clin Sci (Lond). 2018. PMID: 30219798
-
Notch signaling inhibitor DAPT provides protection against acute craniocerebral injury.PLoS One. 2018 Feb 15;13(2):e0193037. doi: 10.1371/journal.pone.0193037. eCollection 2018. PLoS One. 2018. PMID: 29447233 Free PMC article.
-
Notch signaling in pulmonary hypertension.Adv Exp Med Biol. 2010;661:279-98. doi: 10.1007/978-1-60761-500-2_18. Adv Exp Med Biol. 2010. PMID: 20204737 Review.
-
Molecular Mechanisms Underlying Remodeling of Ductus Arteriosus: Looking beyond the Prostaglandin Pathway.Int J Mol Sci. 2021 Mar 22;22(6):3238. doi: 10.3390/ijms22063238. Int J Mol Sci. 2021. PMID: 33810164 Free PMC article. Review.
Cited by
-
The Use of Nutraceuticals to Counteract Atherosclerosis: The Role of the Notch Pathway.Oxid Med Cell Longev. 2019 May 2;2019:5470470. doi: 10.1155/2019/5470470. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31915510 Free PMC article. Review.
-
Dynamic transcriptome and histomorphology analysis of developmental traits of hindlimb thigh muscle from Odorrana tormota and its adaptability to different life history stages.BMC Genomics. 2021 May 20;22(1):369. doi: 10.1186/s12864-021-07677-0. BMC Genomics. 2021. PMID: 34016051 Free PMC article.
-
Elastin-driven genetic diseases.Matrix Biol. 2018 Oct;71-72:144-160. doi: 10.1016/j.matbio.2018.02.021. Epub 2018 Feb 28. Matrix Biol. 2018. PMID: 29501665 Free PMC article. Review.
-
Modeling CADASIL vascular pathologies with patient-derived induced pluripotent stem cells.Protein Cell. 2019 Apr;10(4):249-271. doi: 10.1007/s13238-019-0608-1. Epub 2019 Feb 18. Protein Cell. 2019. PMID: 30778920 Free PMC article.
-
Relationship between Angiotensin II, Vascular Endothelial Growth Factor, and Arteriosclerosis Obliterans.Dis Markers. 2023 Feb 21;2023:1316821. doi: 10.1155/2023/1316821. eCollection 2023. Dis Markers. 2023. PMID: 36865500 Free PMC article.
References
-
- Smith GC. The pharmacology of the ductus arteriosus. Pharmacol Rev. 1998;50:35–58. - PubMed
-
- Hermes-DeSantis ER, Clyman RI. Patent ductus arteriosus: pathophysiology and management. J Perinatol. 2006;26(Suppl 1):S14–S18. - PubMed
-
- Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K. et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–81. - PubMed
-
- Little DC, Pratt TC, Blalock SE, Krauss DR, Cooney DR, Custer MD. Patent ductus arteriosus in micropreemies and full-term infants: the relative merits of surgical ligation versus indomethacin treatment. J Pediatr Surg. 2003;38:492–6. - PubMed
-
- Coceani F, Baragatti B. Mechanisms for ductus arteriosus closure. Semin Perinatol. 2012;36:92–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

