Kaposi's Sarcoma Herpesvirus Genome Persistence
- PMID: 27570517
- PMCID: PMC4982378
- DOI: 10.3389/fmicb.2016.01149
Kaposi's Sarcoma Herpesvirus Genome Persistence
Abstract
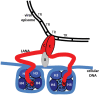
Kaposi's sarcoma-associated herpesvirus (KSHV) has an etiologic role in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. These diseases are most common in immunocompromised individuals, especially those with AIDS. Similar to all herpesviruses, KSHV infection is lifelong. KSHV infection in tumor cells is primarily latent, with only a small subset of cells undergoing lytic infection. During latency, the KSHV genome persists as a multiple copy, extrachromosomal episome in the nucleus. In order to persist in proliferating tumor cells, the viral genome replicates once per cell cycle and then segregates to daughter cell nuclei. KSHV only expresses several genes during latent infection. Prominent among these genes, is the latency-associated nuclear antigen (LANA). LANA is responsible for KSHV genome persistence and also exerts transcriptional regulatory effects. LANA mediates KSHV DNA replication and in addition, is responsible for segregation of replicated genomes to daughter nuclei. LANA serves as a molecular tether, bridging the viral genome to mitotic chromosomes to ensure that KSHV DNA reaches progeny nuclei. N-terminal LANA attaches to mitotic chromosomes by binding histones H2A/H2B at the surface of the nucleosome. C-terminal LANA binds specific KSHV DNA sequence and also has a role in chromosome attachment. In addition to the essential roles of N- and C-terminal LANA in genome persistence, internal LANA sequence is also critical for efficient episome maintenance. LANA's role as an essential mediator of virus persistence makes it an attractive target for inhibition in order to prevent or treat KSHV infection and disease.
Keywords: DNA binding; KSHV; chromosome; latency-associated nuclear antigen; viral persistence.
Figures






Similar articles
-
Kaposi's Sarcoma-Associated Herpesvirus LANA-Adjacent Regions with Distinct Functions in Episome Segregation or Maintenance.J Virol. 2019 Mar 5;93(6):e02158-18. doi: 10.1128/JVI.02158-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626680 Free PMC article.
-
Identification of Kaposi's sarcoma-associated herpesvirus LANA regions important for episome segregation, replication, and persistence.J Virol. 2013 Nov;87(22):12270-83. doi: 10.1128/JVI.01243-13. Epub 2013 Sep 4. J Virol. 2013. PMID: 24006437 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus LANA hitches a ride on the chromosome.Cell Cycle. 2006 May;5(10):1048-52. doi: 10.4161/cc.5.10.2768. Epub 2006 May 15. Cell Cycle. 2006. PMID: 16721045
-
The latency-associated nuclear antigen, a multifunctional protein central to Kaposi's sarcoma-associated herpesvirus latency.Future Microbiol. 2011 Dec;6(12):1399-413. doi: 10.2217/fmb.11.137. Future Microbiol. 2011. PMID: 22122438 Free PMC article. Review.
-
KSHV Genome Replication and Maintenance.Front Microbiol. 2016 Feb 1;7:54. doi: 10.3389/fmicb.2016.00054. eCollection 2016. Front Microbiol. 2016. PMID: 26870016 Free PMC article. Review.
Cited by
-
Experience with Kaposi Sarcoma Herpesvirus Inflammatory Cytokine Syndrome in a Large Urban HIV Clinic in the United States: Case Series and Literature Review.Open Forum Infect Dis. 2017 Sep 13;4(4):ofx196. doi: 10.1093/ofid/ofx196. eCollection 2017 Fall. Open Forum Infect Dis. 2017. PMID: 29766014 Free PMC article. Review.
-
Targeted delivery of miR-34a-5p by phenylborate-coupled polyethylenimide nanocarriers for anti-KSHV treatment.Front Bioeng Biotechnol. 2024 Jan 8;11:1343956. doi: 10.3389/fbioe.2023.1343956. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38260739 Free PMC article.
-
Herpesviruses: overview of systematics, genomic complexity and life cycle.Virol J. 2025 May 22;22(1):155. doi: 10.1186/s12985-025-02779-7. Virol J. 2025. PMID: 40399963 Free PMC article. Review.
-
NDRG1 facilitates lytic replication of Kaposi's sarcoma-associated herpesvirus by maintaining the stability of the KSHV helicase.PLoS Pathog. 2021 Jun 2;17(6):e1009645. doi: 10.1371/journal.ppat.1009645. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34077484 Free PMC article.
-
An Update on the Metabolic Landscape of Oncogenic Viruses.Cancers (Basel). 2022 Nov 23;14(23):5742. doi: 10.3390/cancers14235742. Cancers (Basel). 2022. PMID: 36497226 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

