New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation
- PMID: 27570525
- PMCID: PMC4981595
- DOI: 10.3389/fimmu.2016.00302
New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation
Abstract
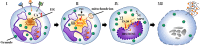
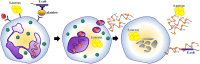
Recent data suggest that NETosis plays a crucial role in the innate immune response and disturbs the homeostasis of the immune system. NETosis is a form of neutrophil-specific cell death characterized by the release of large web-like structures referred to as neutrophil extracellular traps (NETs). NETs are composed of DNA strands associated with histones and decorated with about 20 different proteins, including neutrophil elastase, myeloperoxidase, cathepsin G, proteinase 3, high mobility group protein B1, and LL37. Reportedly, NETosis can be induced by several microbes, and particulate matter including sterile stimuli, via distinct cellular mechanisms. Meanwhile, suicidal NETosis and vital NETosis are controversial. As we enter the second decade of research on NETosis, we have partly understood NETs as double-edged swords of innate immunity. In this review, we will discuss the mechanisms of NETosis, its antimicrobial action, and role in autoimmune diseases, as well as the relatively new field of NET-associated mitochondrial DNA.
Keywords: NETosis; NETs; antimicrobial activity; autoimmune diseases; mitochondrial DNA.
Figures
References
-
- Takei H, Araki A, Watanabe H, Ichinose A, Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol (1996) 59(2):229–40. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

