New method for sequestration of silver nanoparticles in aqueous media: in route toward municipal wastewater
- PMID: 27570541
- PMCID: PMC5002176
- DOI: 10.1186/s13065-016-0198-4
New method for sequestration of silver nanoparticles in aqueous media: in route toward municipal wastewater
Abstract
Background: Nanomaterials are widely used in industry for their specific properties. Silver nanoparticles (Ag NPs) are largely used in several consumer products notably for their antibacterial properties and will likely be found in wastewater, then in the receiving environment. The development of a product capable to sequestrate those released contaminants is needed. Under environmental conditions, the biopolymer chitosan can generally coordinate the cationic metals. Ag NPs present unique properties due to their high surface/mass ratio which are promising for their sequestration.
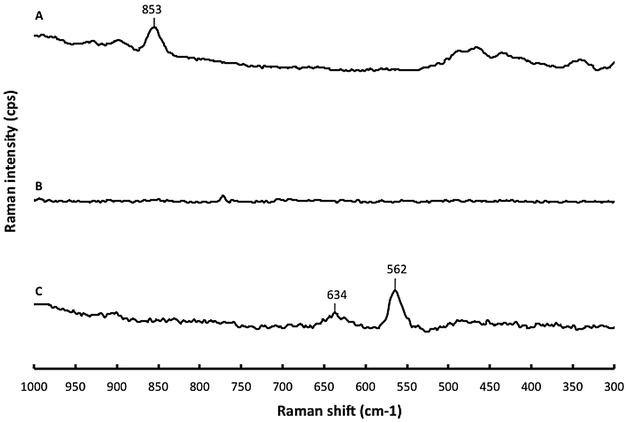
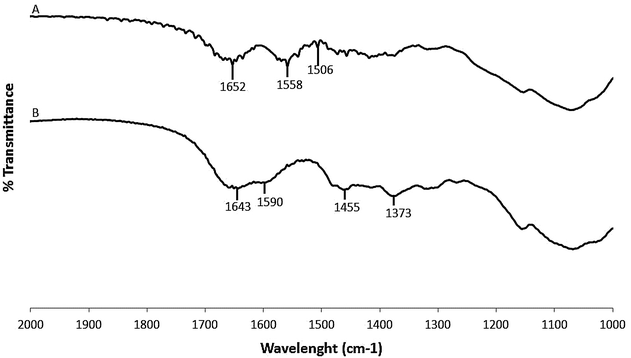
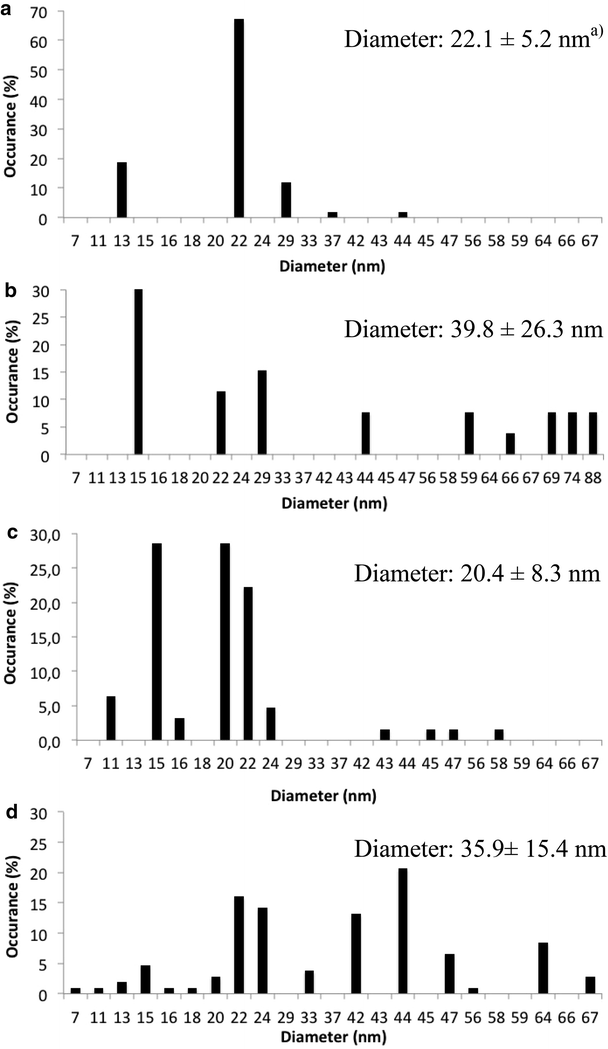
Results: The immobilization of chitosan on functionalized silica assisted by microwaves gives a sequestering agent of silver while being easily recoverable. The IR spectrum confirmed the immobilization of N,N-dimethylchitosan (DMC) on silica core. The immobilized DMC gave a percentage of sequestration of Ag NPs (120 µg L(-1)) in nanopure water of 84.2 % in 4 h. The sequestration efficiency was largely dependent of temperature. By addition of hydrosulfide ions, the percentage of sequestration increased up to 100 %. The immobilized DMC recovered a large portion of silver regardless the speciation (Ag NP or Ag(+)). In wastewater, the immobilized DMC sequestered less Ag NPs (51.7 % in 97 % wastewater). The presence of anionic (sodium dodecyl sulfate and sodium N-lauroylsarcosinate) and non-ionic surfactants (cetyl alcohol) increased the hydrophobicity of Ag NPs and decreased the percentage of sequestration.
Conclusions: The immobilized DMC is a promising tool for sequestrating such emerging pollutant at low concentrations in a large volume of sample that would allow the characterization of concentrated Ag NPs by transmission electron microscopy. The efficiency of the support to sequestrate would likely be influenced by the chemical environment of the Ag NP solution.
Keywords: Ag NP; Organic matter; Removal; Silica; Silver sulfide; Supported polysaccharide; Wastewater.
Figures




References
-
- Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Haggens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van De Meent D, Dekkers S, De Jong WH, Van Zijverden M, Sips AJA, Geertsma RE. Nano-silver—a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3:109–138. doi: 10.1080/17435390902725914. - DOI
-
- Savage N, Diallo MS. Nanomaterials and water purification: opportunities and challenges. J Nanopart Res. 2005;7:331–342. doi: 10.1007/s11051-005-7523-5. - DOI
-
- Guidal E. Heterogenous catalysis on chitosan-based materials: a review. Prog Polym Sci. 2005;30:71–109. doi: 10.1016/j.progpolymsci.2004.12.001. - DOI
-
- Piccino F, Gottschalk F, Seeger S, Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res. 2012;14:1109–1120. doi: 10.1007/s11051-012-1109-9. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

