Polydopamine Coated Single-Walled Carbon Nanotubes as a Versatile Platform with Radionuclide Labeling for Multimodal Tumor Imaging and Therapy
- PMID: 27570554
- PMCID: PMC4997240
- DOI: 10.7150/thno.16047
Polydopamine Coated Single-Walled Carbon Nanotubes as a Versatile Platform with Radionuclide Labeling for Multimodal Tumor Imaging and Therapy
Abstract
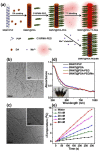
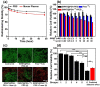
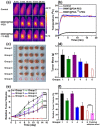
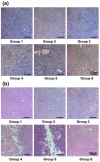
Single-walled carbon nanotubes (SWNTs) with various unique properties have attracted great attention in cancer theranostics. Herein, SWNTs are coated with a shell of polydopamine (PDA), which is further modified by polyethylene glycol (PEG). The PDA shell in the obtained SWNT@PDA-PEG could chelate Mn(2+), which together with metallic nanoparticulate impurities anchored on SWNTs offer enhanced both T1 and T2 contrasts under magnetic resonance (MR) imaging. Meanwhile, also utilizing the PDA shell, radionuclide (131)I could be easily labeled onto SWNT@PDA-PEG, enabling nuclear imaging and radioisotope cancer therapy. As revealed by MR & gamma imaging, efficient tumor accumulation of SWNT@PDA-(131)I-PEG is observed after systemic administration into mice. By further utilizing the strong near-infarared (NIR) absorbance of SWNTs, NIR-triggered photothermal therapy in combination with (131)I-based radioisotope therapy is realized in our animal experiments, in which a remarkable synergistic antitumor therapeutic effect is observed compared to monotherapies. Our work not only presents a new type of theranostic nanoplatform based on SWNTs, but also suggests the promise of PDA coating as a general approach to modify nano-agents and endow them with highly integrated functionalities.
Keywords: Combined therapy; Multimodal imaging; Polydopamine; Radiolabeling; Single-walled carbon nanotubes.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures


References
-
- Zhong J, Wen L, Yang S, Xiang L, Chen Q, Xing D. Imaging-guided high-efficient photoacoustic tumor therapy with targeting gold nanorods. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11:1499–509. - PubMed
-
- Zhong J, Yang S, Wen L, Xing D. Imaging-guided photoacoustic drug release and synergistic chemo-photoacoustic therapy with paclitaxel-containing nanoparticles. Journal of Controlled Release. 2016;226:77–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

