Antibodies inside of a cell can change its outside: Can intrabodies provide a new therapeutic paradigm?
- PMID: 27570612
- PMCID: PMC4990636
- DOI: 10.1016/j.csbj.2016.07.003
Antibodies inside of a cell can change its outside: Can intrabodies provide a new therapeutic paradigm?
Abstract
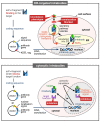
Challenges posed by complex diseases such as cancer, chronic viral infections, neurodegenerative disorders and many others have forced researchers to think beyond classic small molecule drugs, exploring new therapeutic strategies such as therapy with RNAi, CRISPR/Cas9 or antibody therapies as single or as combination therapies with existing drugs. While classic antibody therapies based on parenteral application can only reach extracellular targets, intracellular application of antibodies could provide specific advantages but is so far little recognized in translational research. Intrabodies allow high specificity and targeting of splice variants or post translational modifications. At the same time off target effects can be minimized by thorough biochemical characterization. Knockdown of cellular proteins by intrabodies has been reported for a significant number of disease-relevant targets, including ErbB-2, EGFR, VEGFR-2, Metalloproteinase MMP2 and MMP9, β-amyloid protein, α-synuclein, HIV gp120, HCV core and many others. This review outlines the recent advances in ER intrabody technology and their potential use in therapy.
Figures
Similar articles
-
Specific in vivo knockdown of protein function by intrabodies.MAbs. 2015;7(6):1010-35. doi: 10.1080/19420862.2015.1076601. Epub 2015 Aug 7. MAbs. 2015. PMID: 26252565 Free PMC article. Review.
-
Recent Advances with ER Targeted Intrabodies.Adv Exp Med Biol. 2016;917:77-93. doi: 10.1007/978-3-319-32805-8_5. Adv Exp Med Biol. 2016. PMID: 27236553 Review.
-
Blocking translocation of cell surface molecules from the ER to the cell surface by intracellular antibodies targeted to the ER.J Cell Mol Med. 2007 Jan-Feb;11(1):54-70. doi: 10.1111/j.1582-4934.2007.00002.x. J Cell Mol Med. 2007. PMID: 17367501 Free PMC article. Review.
-
Making cell-permeable antibodies (Transbody) through fusion of protein transduction domains (PTD) with single chain variable fragment (scFv) antibodies: potential advantages over antibodies expressed within the intracellular environment (Intrabody).Med Hypotheses. 2005;64(6):1105-8. doi: 10.1016/j.mehy.2005.01.011. Med Hypotheses. 2005. PMID: 15823695
-
Intracellular antibodies (intrabodies) and their therapeutic potential.Handb Exp Pharmacol. 2008;(181):343-73. doi: 10.1007/978-3-540-73259-4_15. Handb Exp Pharmacol. 2008. PMID: 18071953 Review.
Cited by
-
Porcine parvovirus VP1/VP2 on a time series epitope mapping: exploring the effects of high hydrostatic pressure on the immune recognition of antigens.Virol J. 2019 Jun 3;16(1):75. doi: 10.1186/s12985-019-1165-1. Virol J. 2019. PMID: 31159841 Free PMC article.
-
pH regulators to target the tumor immune microenvironment in human hepatocellular carcinoma.Oncoimmunology. 2018 Mar 26;7(7):e1445452. doi: 10.1080/2162402X.2018.1445452. eCollection 2018. Oncoimmunology. 2018. PMID: 29900055 Free PMC article.
-
Intrabody against prolyl hydroxylase 2 promotes angiogenesis by stabilizing hypoxia-inducible factor-1α.Sci Rep. 2019 Aug 14;9(1):11861. doi: 10.1038/s41598-019-47891-1. Sci Rep. 2019. PMID: 31413262 Free PMC article.
-
Engineered Extracellular Vesicles/Exosomes as a New Tool against Neurodegenerative Diseases.Pharmaceutics. 2020 Jun 9;12(6):529. doi: 10.3390/pharmaceutics12060529. Pharmaceutics. 2020. PMID: 32526949 Free PMC article. Review.
-
Therapeutic Antibodies against Intracellular Tumor Antigens.Front Immunol. 2017 Aug 18;8:1001. doi: 10.3389/fimmu.2017.01001. eCollection 2017. Front Immunol. 2017. PMID: 28868054 Free PMC article. Review.
References
-
- Accardi L., Paolini F., Mandarino A., Percario Z., Di Bonito P., Di Carlo V. In vivo antitumor effect of an intracellular single-chain antibody fragment against the E7 oncoprotein of human papillomavirus 16. Int J Cancer. 2014;134(11):2742–2747. - PubMed
-
- Auf der Maur A., Tissot K., Barberis A. Antigen-independent selection of intracellular stable antibody frameworks. Methods. 2004;34(2):215–224. - PubMed
-
- Backhaus O., Böldicke T. ER-targeted intrabodies mediating specific in vivo knockdown of transitory proteins in comparison to RNAi. In: D. I. Y. A., editor. RNA interference. InTech; 2016.
-
- Behr J.P. The proton sponge: A trick to enter cells the viruses did not exploit. Chimia. 1997;51(1/2):34–36.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
