Single Nanoparticle Plasmonic Spectroscopy for Study of the Efflux Function of Multidrug ABC Membrane Transporters of Single Live Cells
- PMID: 27570617
- PMCID: PMC4999259
- DOI: 10.1039/C6RA05895G
Single Nanoparticle Plasmonic Spectroscopy for Study of the Efflux Function of Multidrug ABC Membrane Transporters of Single Live Cells
Abstract
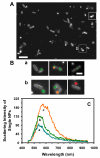
ATP-binding cassette (ABC) membrane transporters exist in all living organisms and play key roles in a wide range of cellular and physiological functions. The ABC transporters can selectively extrude a wide variety of structurally and functionally unrelated substrates, leading to multidrug resistance. Despite extensive study, their efflux molecular mechanisms remain elusive. In this study, we synthesized and characterized purified silver nanoparticles (Ag NPs) (97 ± 13 nm in diameter), and used them as photostable optical imaging probes to study efflux kinetics of ABC membrane transporters (BmrA) of single live cells (B. subtillis). The NPs with concentrations up to 3.7 pM were stable (non-aggregated) in a PBS buffer and biocompatible with the cells. We found a high dependence of accumulation of the intracellular NPs in single live cells (WT, Ct-BmrA-EGFP, ΔbmrA) upon the cellular expression level of BmrA and NP concentration (0.93, 1.85 and 3.7 pM), showing the highest accumulation of intracellular NPs in ΔbmrA (deletion of BmrA) and the lowest ones in Ct-BmrA-EGFP (over-expression of BmrA). Interestingly, the accumulation of intracellular NPs in ΔbmrA increases nearly proportionally with the NP concentration, while those in WT and Ct-BrmA-EGFP do not. This suggests that the NPs enter the cells via passive diffusion driven by concentration gradients and are extruded out of cells by BmrA transporters, similar to conventional pump substrates (antibiotics). This study shows that such large substrates (84-100 nm NPs) can enter into the live cells and be extruded out of the cells by BmrA, and the NPs can serve as nm-sized optical imaging probes to study the size-dependent efflux kinetics of membrane transporters in single live cells in real time.
Keywords: ABC (BmrA) transporter; Ag nanoparticle; Bacillus subtilis; multidrug resistance; single cell imaging; single nanoparticle plasmonic spectroscopy.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
