Multiphoton microscopy with clearing for three dimensional histology of kidney biopsies
- PMID: 27570700
- PMCID: PMC4986816
- DOI: 10.1364/BOE.7.003089
Multiphoton microscopy with clearing for three dimensional histology of kidney biopsies
Abstract
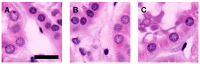
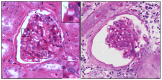
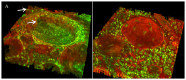
We present a multiphoton microscopy approach with clearing optimized for pathology evaluation producing image quality comparable to traditional histology. Use of benzyl alcohol/benzyl benzoate with 4',6-diamidino-2-phenylindole and eosin in an optimized imaging setup results in optical sections nearly indistinguishable from traditionally-cut sections. Application to human renal tissue demonstrates diagnostic-level image quality can be maintained through 1 millimeter of tissue. Three dimensional perspectives reveal changes of glomerular capsule cells not evident on single sections. Collagen-derived second harmonic generation can be visualized through entire biopsies. Multiphoton microscopy with clearing has potential for increasing the yield of histologic evaluation of biopsy specimens.
Keywords: (180.0180) Microscopy; (180.4315) Nonlinear microscopy; (180.6900) Three-dimensional microscopy.
Figures



References
-
- Zipfel W. R., Williams R. M., Christie R., Nikitin A. Y., Hyman B. T., Webb W. W., “Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation,” Proc. Natl. Acad. Sci. U.S.A. 100(12), 7075–7080 (2003).10.1073/pnas.0832308100 - DOI - PMC - PubMed
-
- Marcussen N., “Atubular glomeruli and the structural basis for chronic renal failure,” Lab. Invest. 66(3), 265–284 (1992). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
