The effect of vitamin D on COPD exacerbation: a double blind randomized placebo-controlled parallel clinical trial
- PMID: 27570748
- PMCID: PMC5002185
- DOI: 10.1186/s40200-016-0257-3
The effect of vitamin D on COPD exacerbation: a double blind randomized placebo-controlled parallel clinical trial
Abstract
Background: To investigate the effect of supplementation of standard treatment (inhaled long-acting β2 agonists, anticholinergics and corticosteroids) with vitamin D on C reactive protein and pulmonary function tests in patients with COPD exacerbation.
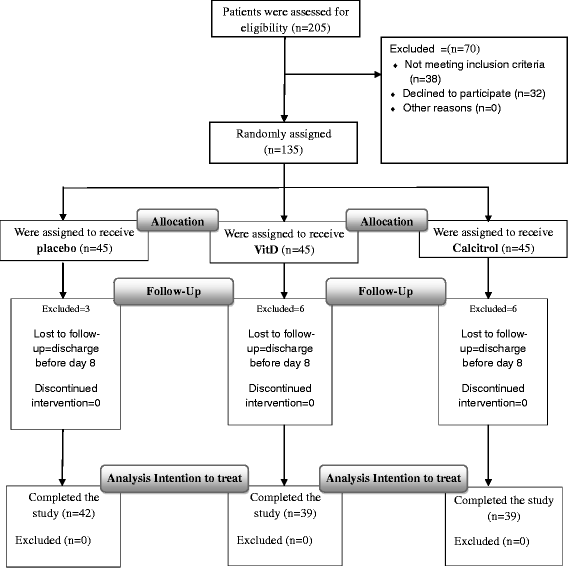
Design: Randomized, single-center, double-blind, placebo-controlled parallel trial. One teaching hospital Participants: 135 patients in pulmonary ward with moderate to severe COPD and exacerbations.120 patients fulfilled the study protocol.
Interventions: Patients were randomly divided into three groups receiving 7 day treatment with 0.25 μg calcitriol daily (n = 45), 50000 IU daily of vitamin D (n = 45) or placebo (n = 45). An independent nurse was responsible for allocation, preparation, and accounting of trial medications.
Main outcome measures: Maximal expiratory flow volume (FEV1) and forced volume capacity curves (FVC) and Modified Medical Research Council (MMRC) scale.
Results: Out of 135 patients who were recruited consecutively, 45 patients randomly were randomly assigned in three groups (balance blocked randomization.15 patients were dropped out due to non-compliance for second PFT. Intention to treat analysis was carried out for 120 participants. The difference between before and after treatment FEV1 and FEV1/FVC ratio had no significant difference between treatment groups and placebo. (P = 0.43, P = 0.51, respectively)but clinical improvement was significant in patients who received calcitriol. No side effects were reported.
Conclusions: Short term treatment with either calcitriol or 25(OH) 2Vit D didn't changed FEV1 or FVC in vitamin D sufficient patients with COPD exacerbation; nevertheless it can provide clinical benefit.
Trial registration: Iranian Registry of Clinical Trials no. IRCT138712271774N1. Registered 10 April 2011.
Keywords: COPD; Calcitriol; Forced Vital Capacity; Forced expiratory volume; Pulmonary function tests; Vitamin D.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous