Axonal Localization of Integrins in the CNS Is Neuronal Type and Age Dependent
- PMID: 27570822
- PMCID: PMC4987411
- DOI: 10.1523/ENEURO.0029-16.2016
Axonal Localization of Integrins in the CNS Is Neuronal Type and Age Dependent
Abstract
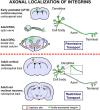
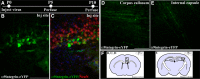
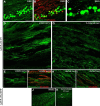
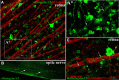
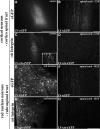
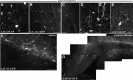
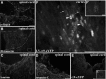
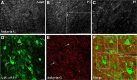
The regenerative ability of CNS axons decreases with age, however, this ability remains largely intact in PNS axons throughout adulthood. These differences are likely to correspond with age-related silencing of proteins necessary for axon growth and elongation. In previous studies, it has been shown that reintroduction of the α9 integrin subunit (tenascin-C receptor, α9) that is downregulated in adult CNS can improve neurite outgrowth and sensory axon regeneration after a dorsal rhizotomy or a dorsal column crush spinal cord lesion. In the current study, we demonstrate that virally expressed integrins (α9, α6, or β1 integrin) in the adult rat sensorimotor cortex and adult red nucleus are excluded from axons following neuronal transduction. Attempts to stimulate transport by inclusion of a cervical spinal injury and thus an upregulation of extracellular matrix molecules at the lesion site, or cotransduction with its binding partner, β1 integrin, did not induce integrin localization within axons. In contrast, virally expressed α9 integrin in developing rat cortex (postnatal day 5 or 10) demonstrated clear localization of integrins in cortical axons revealed by the presence of integrin in the axons of the corpus callosum and internal capsule, as well as in the neuronal cell body. Furthermore, examination of dorsal root ganglia neurons and retinal ganglion cells demonstrated integrin localization both within peripheral nerve as well as dorsal root axons and within optic nerve axons, respectively. Together, our results suggest a differential ability for in vivo axonal transport of transmembrane proteins dependent on neuronal age and subtype.
Keywords: adeno-associated virus; axon initial segment; dorsal root ganglia; integrin; retinal ganglion cell; sensorimotor cortex.
Figures
References
-
- Altman J, Bayer SA (1995) Atlas of prenatal rat development. Boca Raton, FL: CRC Press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
