Differential Contributions of Olfactory Receptor Neurons in a Drosophila Olfactory Circuit
- PMID: 27570823
- PMCID: PMC4987412
- DOI: 10.1523/ENEURO.0045-16.2016
Differential Contributions of Olfactory Receptor Neurons in a Drosophila Olfactory Circuit
Abstract
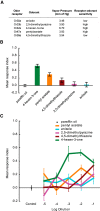
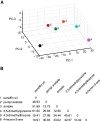
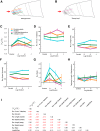
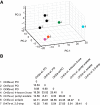
The ability of an animal to detect, discriminate, and respond to odors depends on the functions of its olfactory receptor neurons (ORNs). The extent to which each ORN, upon activation, contributes to chemotaxis is not well understood. We hypothesized that strong activation of each ORN elicits a different behavioral response in the Drosophila melanogaster larva by differentially affecting the composition of its navigational behavior. To test this hypothesis, we exposed Drosophila larvae to specific odorants to analyze the effect of individual ORN activity on chemotaxis. We used two different behavioral paradigms to analyze the chemotaxis response of larvae to odorants. When tested with five different odorants that elicit strong physiological responses from single ORNs, larval behavioral responses toward each odorant differed in the strength of attraction as well as in the composition of discrete navigational elements, such as runs and turns. Further, behavioral responses to odorants did not correlate with either the strength of odor gradients tested or the sensitivity of each ORN to its cognate odorant. Finally, we provide evidence that wild-type larvae with all ORNs intact exhibit higher behavioral variance than mutant larvae that have only a single pair of functional ORNs. We conclude that individual ORNs contribute differently to the olfactory circuit that instructs chemotactic responses. Our results, along with recent studies from other groups, suggest that ORNs are functionally nonequivalent units. These results have implications for understanding peripheral odor coding.
Keywords: Drosophila; behavior; larva; odor receptor; olfaction; receptor neuron.
Figures


References
-
- Andersson MN, Schlyter F, Hill SR, Dekker T (2012) What reaches the antenna? How to calibrate odor flux and ligand-receptor affinities. Chem Senses 37:403–420. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
