Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model
- PMID: 27570911
- PMCID: PMC5073241
- DOI: 10.1089/ten.TEA.2016.0134
Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model
Abstract
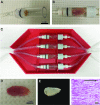
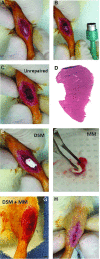
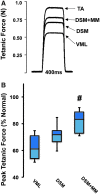
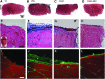
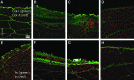
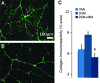
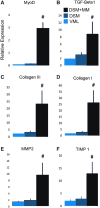
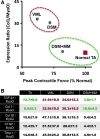
Skeletal muscle is capable of robust self-repair following mild trauma, yet in cases of traumatic volumetric muscle loss (VML), where more than 20% of a muscle's mass is lost, this capacity is overwhelmed. Current autogenic whole muscle transfer techniques are imperfect, which has motivated the exploration of implantable scaffolding strategies. In this study, the use of an allogeneic decellularized skeletal muscle (DSM) scaffold with and without the addition of minced muscle (MM) autograft tissue was explored as a repair strategy using a lower-limb VML injury model (n = 8/sample group). We found that the repair of VML injuries using DSM + MM scaffolds significantly increased recovery of peak contractile force (81 ± 3% of normal contralateral muscle) compared to unrepaired VML controls (62 ± 4%). Similar significant improvements were measured for restoration of muscle mass (88 ± 3%) in response to DSM + MM repair compared to unrepaired VML controls (79 ± 3%). Histological findings revealed a marked decrease in collagen dense repair tissue formation both at and away from the implant site for DSM + MM repaired muscles. The addition of MM to DSM significantly increased MyoD expression, compared to isolated DSM treatment (21-fold increase) and unrepaired VML (37-fold) controls. These findings support the further exploration of both DSM and MM as promising strategies for the repair of VML injury.
Conflict of interest statement
Statement No competing financial interests exist.
Figures

Similar articles
-
Cell-Derived Extracellular Matrix Fiber Scaffolds Improve Recovery from Volumetric Muscle Loss.Tissue Eng Part A. 2024 Mar;30(5-6):181-191. doi: 10.1089/ten.TEA.2022.0227. Epub 2023 Nov 21. Tissue Eng Part A. 2024. PMID: 37658842
-
Regenerative Repair of Volumetric Muscle Loss Injury is Sensitive to Age.Tissue Eng Part A. 2020 Jan;26(1-2):3-14. doi: 10.1089/ten.TEA.2019.0034. Epub 2019 Aug 9. Tissue Eng Part A. 2020. PMID: 31064280 Free PMC article.
-
Recovery from volumetric muscle loss injury: A comparison between young and aged rats.Exp Gerontol. 2016 Oct;83:37-46. doi: 10.1016/j.exger.2016.07.008. Epub 2016 Jul 17. Exp Gerontol. 2016. PMID: 27435497 Free PMC article.
-
Extracellular matrix scaffolds for treatment of large volume muscle injuries: A review.Vet Surg. 2018 May;47(4):524-535. doi: 10.1111/vsu.12787. Epub 2018 Mar 30. Vet Surg. 2018. PMID: 29603757 Review.
-
Decellularized Tissue for Muscle Regeneration.Int J Mol Sci. 2018 Aug 14;19(8):2392. doi: 10.3390/ijms19082392. Int J Mol Sci. 2018. PMID: 30110909 Free PMC article. Review.
Cited by
-
Recent Trends in Injury Models to Study Skeletal Muscle Regeneration and Repair.Bioengineering (Basel). 2020 Jul 20;7(3):76. doi: 10.3390/bioengineering7030076. Bioengineering (Basel). 2020. PMID: 32698352 Free PMC article. Review.
-
Vascularized and Innervated Skeletal Muscle Tissue Engineering.Adv Healthc Mater. 2020 Jan;9(1):e1900626. doi: 10.1002/adhm.201900626. Epub 2019 Oct 17. Adv Healthc Mater. 2020. PMID: 31622051 Free PMC article. Review.
-
Application of decellularization-recellularization technique in plastic and reconstructive surgery.Chin Med J (Engl). 2023 Sep 5;136(17):2017-2027. doi: 10.1097/CM9.0000000000002085. Chin Med J (Engl). 2023. PMID: 36752783 Free PMC article. Review.
-
Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges.Materials (Basel). 2018 Jun 29;11(7):1116. doi: 10.3390/ma11071116. Materials (Basel). 2018. PMID: 29966303 Free PMC article. Review.
-
Biomimetic sponges improve muscle structure and function following volumetric muscle loss.J Biomed Mater Res A. 2021 Nov;109(11):2280-2293. doi: 10.1002/jbm.a.37212. Epub 2021 May 7. J Biomed Mater Res A. 2021. PMID: 33960118 Free PMC article.
References
-
- Terada N., Takayama S., Yamada H., and Seki T. Muscle repair after a transsection injury with development of a gap: an experimental study in rats. Scand J Plast Reconstr Surg Hand Surg 35 , 233, 2001. - PubMed
-
- Oishi S.N., and Ezaki M. Free gracilis transfer to restore finger flexion in Volkmann ischemic contracture. Tech Hand Up Extrem Surg 14 , 104, 2010. - PubMed
-
- Terzis J.K., and Kostopoulos V.K. Free muscle transfer in posttraumatic plexopathies: part 1: the shoulder. Ann Plast Surg 65 , 312, 2010. - PubMed
-
- Vekris M.D., Beris A.E., Lykissas M.G., Korompilias A.V., Vekris A.D., and Soucacos P.N. Restoration of elbow function in severe brachial plexus paralysis via muscle transfers. Injury 39 Suppl 3 , S15, 2008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

