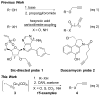
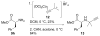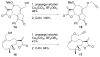Alkyne Ligation Handles: Propargylation of Hydroxyl, Sulfhydryl, Amino, and Carboxyl Groups via the Nicholas Reaction
- PMID: 27570975
- PMCID: PMC5234733
- DOI: 10.1021/acs.orglett.6b02088
Alkyne Ligation Handles: Propargylation of Hydroxyl, Sulfhydryl, Amino, and Carboxyl Groups via the Nicholas Reaction
Abstract
The Nicholas reaction has been applied to the installation of alkyne ligation handles. Acid-promoted propargylation of hydroxyl, sulfhydryl, amino, and carboxyl groups using dicobalt hexacarbonyl-stabilized propargylium ions is reported. This method is useful for introduction of propargyl groups into base-sensitive molecules, thereby expanding the toolbox of methods for the incorporation of alkynes for bio-orthogonal reactions. High-value molecules are used as the limiting reagent, and various propargylium ion precursors are compared.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Mu2-alkyne dicobalt(0)hexacarbonyl complexes as carbon monoxide-releasing molecules (CO-RMs): probing the release mechanism.Dalton Trans. 2009 May 21;(19):3653-6. doi: 10.1039/b904627p. Epub 2009 Mar 26. Dalton Trans. 2009. PMID: 19417926
-
C-Propargylation Overrides O-Propargylation in Reactions of Propargyl Chloride with Primary Alcohols: Rhodium-Catalyzed Transfer Hydrogenation.Angew Chem Int Ed Engl. 2016 Aug 1;55(32):9207-11. doi: 10.1002/anie.201603575. Epub 2016 Jun 20. Angew Chem Int Ed Engl. 2016. PMID: 27321353 Free PMC article.
-
Zinc catalyzed and mediated asymmetric propargylation of trifluoromethyl ketones with a propargyl boronate.J Org Chem. 2013 Apr 19;78(8):3592-615. doi: 10.1021/jo400080y. Epub 2013 Apr 1. J Org Chem. 2013. PMID: 23544787
-
Direct addition of alkynes to imines and related C=N electrophiles: A convenient access to propargylamines.Chem Commun (Camb). 2006 Nov 4;(41):4263-75. doi: 10.1039/b607986p. Epub 2006 Aug 25. Chem Commun (Camb). 2006. PMID: 17047838 Review.
-
New chemistries for chemoselective peptide ligations and the total synthesis of proteins.Curr Opin Chem Biol. 2014 Oct;22:115-21. doi: 10.1016/j.cbpa.2014.09.032. Epub 2014 Oct 6. Curr Opin Chem Biol. 2014. PMID: 25299573 Review.
Cited by
-
Recent Advances in the Synthesis of Propargyl Derivatives, and Their Application as Synthetic Intermediates and Building Blocks.Molecules. 2023 Apr 11;28(8):3379. doi: 10.3390/molecules28083379. Molecules. 2023. PMID: 37110613 Free PMC article. Review.
-
Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions.J Med Chem. 2017 Feb 9;60(3):839-885. doi: 10.1021/acs.jmedchem.6b00788. Epub 2016 Dec 20. J Med Chem. 2017. PMID: 27996267 Free PMC article.
References
-
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–2599. - PubMed
- Meldal M, Tornoe CW. Chem Rev. 2008;108:2952–3015. - PubMed
- Sletten EM, Bertozzi CR. Angew Chem Int Ed. 2009;48:6974–6998. - PMC - PubMed
- Tang W, Becker ML. Chem Soc Rev. 2014;43:7013–7039. - PubMed
- Martell J, Weerapana E. Molecules. 2014;19:1378–1393. - PMC - PubMed
- Tiwari VK, Mishra BB, Mishra KB, Mishra N, Singh AS, Chen X. Chem Rev. 2016;116:3086–3240. - PubMed
-
- Johansson H, Pedersen DS. Eur J Org Chem. 2012:4267–4281.
- Lehmann J, Wright MH, Sieber SA. Chem Eur J. 2016;22:4666–4678. - PubMed
-
-
For selected examples, see: Bottcher T, Sieber SA. J Am Chem Soc. 2010;132:6964–6972.Kalesh KA, Sim DSB, Wang J, Liu K, Lin Q, Yoa SQ. Chem Commun. 2010;46:1118–1120.Krysiak JM, Kreuzer J, Macheroux P, Hermetter A, Sieber SA, Breinbauer R. Angew Chem Int Ed. 2012;51:7035–7040.Kreuzer J, Bach NC, Forler D, Sieber SA. Chem Sci. 2015;6:237–245.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources