Cardiac Meets Skeletal: What's New in Microfluidic Models for Muscle Tissue Engineering
- PMID: 27571058
- PMCID: PMC6274098
- DOI: 10.3390/molecules21091128
Cardiac Meets Skeletal: What's New in Microfluidic Models for Muscle Tissue Engineering
Abstract
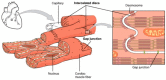
In the last few years microfluidics and microfabrication technique principles have been extensively exploited for biomedical applications. In this framework, organs-on-a-chip represent promising tools to reproduce key features of functional tissue units within microscale culture chambers. These systems offer the possibility to investigate the effects of biochemical, mechanical, and electrical stimulations, which are usually applied to enhance the functionality of the engineered tissues. Since the functionality of muscle tissues relies on the 3D organization and on the perfect coupling between electrochemical stimulation and mechanical contraction, great efforts have been devoted to generate biomimetic skeletal and cardiac systems to allow high-throughput pathophysiological studies and drug screening. This review critically analyzes microfluidic platforms that were designed for skeletal and cardiac muscle tissue engineering. Our aim is to highlight which specific features of the engineered systems promoted a typical reorganization of the engineered construct and to discuss how promising design solutions exploited for skeletal muscle models could be applied to improve cardiac tissue models and vice versa.
Keywords: cardiac muscle; electrical stimulation; heart; in vitro 3D model; mechanical stimulation; microfluidic; organ-on-a-chip; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- PubMed Data. [(accessed on 1 May 2016)]; Available online: http://www.ncbi.nlm.nih.gov/pubmed?term=microfluidics.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

