Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation
- PMID: 27571102
- PMCID: PMC5037474
- DOI: 10.3390/toxins8090248
Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-Referral Treatment for Envenomation
Abstract
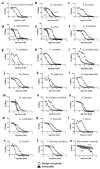
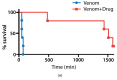
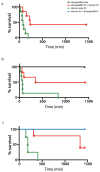
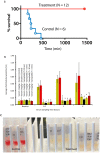
Snakebite remains a neglected medical problem of the developing world with up to 125,000 deaths each year despite more than a century of calls to improve snakebite prevention and care. An estimated 75% of fatalities from snakebite occur outside the hospital setting. Because phospholipase A2 (PLA2) activity is an important component of venom toxicity, we sought candidate PLA2 inhibitors by directly testing drugs. Surprisingly, varespladib and its orally bioavailable prodrug, methyl-varespladib showed high-level secretory PLA2 (sPLA2) inhibition at nanomolar and picomolar concentrations against 28 medically important snake venoms from six continents. In vivo proof-of-concept studies with varespladib had striking survival benefit against lethal doses of Micrurus fulvius and Vipera berus venom, and suppressed venom-induced sPLA2 activity in rats challenged with 100% lethal doses of M. fulvius venom. Rapid development and deployment of a broad-spectrum PLA2 inhibitor alone or in combination with other small molecule inhibitors of snake toxins (e.g., metalloproteases) could fill the critical therapeutic gap spanning pre-referral and hospital setting. Lower barriers for clinical testing of safety tested, repurposed small molecule therapeutics are a potentially economical and effective path forward to fill the pre-referral gap in the setting of snakebite.
Keywords: LY315920; LY333013; antidote; envenomation; field treatment; inhibitor; methyl-varespladib; pre-referral; snakebite; varespladib.
Conflict of interest statement
J.M.: No competing interests; M.L.: Intellectual property including Ophirex stock and salary support; S.S., P.B. have received compensation for consulting including Ophirex stock; J.M. no competing interests. Confirmatory animal studies were performed at a contract research facility (Pacific BioLabs) without author participation; Pilot animal studies were performed by M.L., S.S. and P.B. Yale Center for Molecular Biology (J.M.) acted as a CRO for in vitro studies and authors with competing interests.
Figures

References
-
- Laustsen A.H., Engmark M., Milbo C., Johannesen J., Lomonte B., Gutiérrez J.M., Lohse B. From Fangs to Pharmacology: The Future of Snakebite Envenoming Therapy. Curr. Pharm. Des. 2016;22:1–24. - PubMed
-
- Sharma S.K., Chappuis F., Jha N., Bovier P.A., Loutan L., Koirala S. Impact of snake bites and determinants of fatal outcomes in southeastern Nepal. Am. J. Trop. Med. Hyg. 2004;71:234–238. - PubMed
-
- Casewell N.R., Wagstaff S.C., Wüster W., Cook D.A., Bolton F.M., King S.I., Pla D., Sanz L., Calvete J.J., Harrison R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA. 2014;111:9205–9210. doi: 10.1073/pnas.1405484111. - DOI - PMC - PubMed
-
- Segura A., Herrera M., Villalta M., Vargas M., Uscanga-Reynell A., de León-Rosales S.P., Jiménez-Corona M.E., Reta-Mares J.F., Gutiérrez J.M., León G. Venom of Bothrops asper from Mexico and Costa Rica: Intraspecific variation and cross-neutralization by antivenoms. Toxicon. 2012;59:158–162. doi: 10.1016/j.toxicon.2011.11.005. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

