The Human Connectome Project's neuroimaging approach
- PMID: 27571196
- PMCID: PMC6172654
- DOI: 10.1038/nn.4361
The Human Connectome Project's neuroimaging approach
Abstract
Noninvasive human neuroimaging has yielded many discoveries about the brain. Numerous methodological advances have also occurred, though inertia has slowed their adoption. This paper presents an integrated approach to data acquisition, analysis and sharing that builds upon recent advances, particularly from the Human Connectome Project (HCP). The 'HCP-style' paradigm has seven core tenets: (i) collect multimodal imaging data from many subjects; (ii) acquire data at high spatial and temporal resolution; (iii) preprocess data to minimize distortions, blurring and temporal artifacts; (iv) represent data using the natural geometry of cortical and subcortical structures; (v) accurately align corresponding brain areas across subjects and studies; (vi) analyze data using neurobiologically accurate brain parcellations; and (vii) share published data via user-friendly databases. We illustrate the HCP-style paradigm using existing HCP data sets and provide guidance for future research. Widespread adoption of this paradigm should accelerate progress in understanding the brain in health and disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
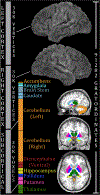
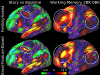
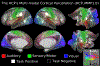
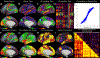

References
-
- Smith SM Introduction to the Neuroimage Special Issue “Mapping the Connectome”. Neuroimage 80, 1 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

