Bile Acid Recognition by NAPE-PLD
- PMID: 27571266
- PMCID: PMC5074845
- DOI: 10.1021/acschembio.6b00624
Bile Acid Recognition by NAPE-PLD
Abstract
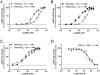
The membrane-associated enzyme NAPE-PLD (N-acyl phosphatidylethanolamine specific-phospholipase D) generates the endogenous cannabinoid arachidonylethanolamide and other lipid signaling amides, including oleoylethanolamide and palmitoylethanolamide. These bioactive molecules play important roles in several physiological pathways including stress and pain response, appetite, and lifespan. Recently, we reported the crystal structure of human NAPE-PLD and discovered specific binding sites for the bile acid deoxycholic acid. In this study, we demonstrate that in the presence of this secondary bile acid, the stiffness of the protein measured by elastic neutron scattering increases, and NAPE-PLD is ∼7 times faster to catalyze the hydrolysis of the more unsaturated substrate N-arachidonyl-phosphatidylethanolamine, compared with N-palmitoyl-phosphatidylethanolamine. Chenodeoxycholic acid and glyco- or tauro-dihydroxy conjugates can also bind to NAPE-PLD and drive its activation. The only natural monohydroxy bile acid, lithocholic acid, shows an affinity of ∼20 μM and acts instead as a reversible inhibitor (IC50 ≈ 68 μM). Overall, these findings provide important insights into the allosteric regulation of the enzyme mediated by bile acid cofactors and reveal that NAPE-PLD responds primarily to the number and position of their hydroxyl groups.
Figures
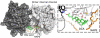


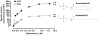
References
-
- Wymann MP, Schneiter R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008;9:162–176. - PubMed
-
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003;4:873–884. - PubMed
-
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. - PubMed
-
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. - PubMed
-
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

