Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats
- PMID: 27571347
- PMCID: PMC5293535
- DOI: 10.1038/nm.4162
Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats
Abstract
Orthopedic implants containing biodegradable magnesium have been used for fracture repair with considerable efficacy; however, the underlying mechanisms by which these implants improve fracture healing remain elusive. Here we show the formation of abundant new bone at peripheral cortical sites after intramedullary implantation of a pin containing ultrapure magnesium into the intact distal femur in rats. This response was accompanied by substantial increases of neuronal calcitonin gene-related polypeptide-α (CGRP) in both the peripheral cortex of the femur and the ipsilateral dorsal root ganglia (DRG). Surgical removal of the periosteum, capsaicin denervation of sensory nerves or knockdown in vivo of the CGRP-receptor-encoding genes Calcrl or Ramp1 substantially reversed the magnesium-induced osteogenesis that we observed in this model. Overexpression of these genes, however, enhanced magnesium-induced osteogenesis. We further found that an elevation of extracellular magnesium induces magnesium transporter 1 (MAGT1)-dependent and transient receptor potential cation channel, subfamily M, member 7 (TRPM7)-dependent magnesium entry, as well as an increase in intracellular adenosine triphosphate (ATP) and the accumulation of terminal synaptic vesicles in isolated rat DRG neurons. In isolated rat periosteum-derived stem cells, CGRP induces CALCRL- and RAMP1-dependent activation of cAMP-responsive element binding protein 1 (CREB1) and SP7 (also known as osterix), and thus enhances osteogenic differentiation of these stem cells. Furthermore, we have developed an innovative, magnesium-containing intramedullary nail that facilitates femur fracture repair in rats with ovariectomy-induced osteoporosis. Taken together, these findings reveal a previously undefined role of magnesium in promoting CGRP-mediated osteogenic differentiation, which suggests the therapeutic potential of this ion in orthopedics.
Figures

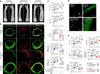
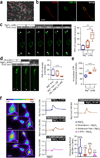
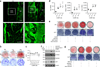


Comment in
-
Bone: Neuronal origin of osteogenic effects of magnesium.Nat Rev Endocrinol. 2016 Dec;12(12):687. doi: 10.1038/nrendo.2016.160. Epub 2016 Sep 16. Nat Rev Endocrinol. 2016. PMID: 27636728 No abstract available.
References
-
- Cheung WH, Chin WC, Qin L, Leung KS. Low intensity pulsed ultrasound enhances fracture healing in both ovariectomy-induced osteoporotic and age-matched normal bones. J. Orthop. Res. 2012;30:129–136. - PubMed
-
- Hayes JS, Richards RG. The use of titanium and stainless steel in fracture fixation. Expert Rev. Med. Devices. 2010;7:843–853. - PubMed
-
- Gu XN, Xie XH, Li N, Zheng YF, Qin L. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012;8:2360–2374. - PubMed
-
- de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol. Rev. 2015;95:1–46. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

