LYN- and AIRE-mediated tolerance checkpoint defects synergize to trigger organ-specific autoimmunity
- PMID: 27571405
- PMCID: PMC5087700
- DOI: 10.1172/JCI84440
LYN- and AIRE-mediated tolerance checkpoint defects synergize to trigger organ-specific autoimmunity
Abstract
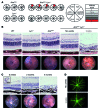
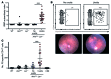
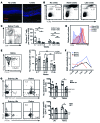
Studies of the genetic factors associated with human autoimmune disease suggest a multigenic origin of susceptibility; however, how these factors interact and through which tolerance pathways they operate generally remain to be defined. One key checkpoint occurs through the activity of the autoimmune regulator AIRE, which promotes central T cell tolerance. Recent reports have described a variety of dominant-negative AIRE mutations that likely contribute to human autoimmunity to a greater extent than previously thought. In families with these mutations, the penetrance of autoimmunity is incomplete, suggesting that other checkpoints play a role in preventing autoimmunity. Here, we tested whether a defect in LYN, an inhibitory protein tyrosine kinase that is implicated in systemic autoimmunity, could combine with an Aire mutation to provoke organ-specific autoimmunity. Indeed, mice with a dominant-negative allele of Aire and deficiency in LYN spontaneously developed organ-specific autoimmunity in the eye. We further determined that a small pool of retinal protein-specific T cells escaped thymic deletion as a result of the hypomorphic Aire function and that these cells also escaped peripheral tolerance in the presence of LYN-deficient dendritic cells, leading to highly destructive autoimmune attack. These findings demonstrate how 2 distinct tolerance pathways can synergize to unleash autoimmunity and have implications for the genetic susceptibility of autoimmune disease.
Figures


Similar articles
-
Autoimmune regulator controls T cell help for pathogenetic autoantibody production in collagen-induced arthritis.Arthritis Rheum. 2009 Jun;60(6):1683-93. doi: 10.1002/art.24501. Arthritis Rheum. 2009. PMID: 19479827
-
Gene dosage--limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity.J Exp Med. 2004 Oct 18;200(8):1015-26. doi: 10.1084/jem.20040581. J Exp Med. 2004. PMID: 15492124 Free PMC article.
-
Limited peripheral T cell anergy predisposes to retinal autoimmunity.J Immunol. 2007 Apr 1;178(7):4276-83. doi: 10.4049/jimmunol.178.7.4276. J Immunol. 2007. PMID: 17371984
-
Recent insights into the role and molecular mechanisms of the autoimmune regulator (AIRE) gene in autoimmunity.Autoimmun Rev. 2011 Jan;10(3):137-43. doi: 10.1016/j.autrev.2010.08.019. Epub 2010 Sep 17. Autoimmun Rev. 2011. PMID: 20850570 Review.
-
Disruption of immunological tolerance: role of AIRE gene in autoimmunity.Autoimmun Rev. 2006 Feb;5(2):145-7. doi: 10.1016/j.autrev.2005.09.001. Epub 2005 Sep 13. Autoimmun Rev. 2006. PMID: 16431348 Review.
Cited by
-
Comparative Analysis of Global Proteome and Lysine Acetylome Between Naive CD4+ T Cells and CD4+ T Follicular Helper Cells.Front Immunol. 2021 Mar 25;12:643441. doi: 10.3389/fimmu.2021.643441. eCollection 2021. Front Immunol. 2021. PMID: 33841426 Free PMC article.
-
Tracking the role of Aire in immune tolerance to the eye with a TCR transgenic mouse model.Proc Natl Acad Sci U S A. 2024 Jan 30;121(5):e2311487121. doi: 10.1073/pnas.2311487121. Epub 2024 Jan 23. Proc Natl Acad Sci U S A. 2024. PMID: 38261611 Free PMC article.
-
Commensal microbiota as a potential trigger of autoimmune uveitis.Expert Rev Clin Immunol. 2017 Apr;13(4):291-293. doi: 10.1080/1744666X.2017.1288098. Epub 2017 Feb 15. Expert Rev Clin Immunol. 2017. PMID: 28145784 Free PMC article. No abstract available.
-
Microbiome and Autoimmune Uveitis.Front Immunol. 2019 Feb 19;10:232. doi: 10.3389/fimmu.2019.00232. eCollection 2019. Front Immunol. 2019. PMID: 30837991 Free PMC article. Review.
-
Autoimmune Regulator is required in female mice for optimal embryonic development and implantation†.Biol Reprod. 2019 Jun 1;100(6):1492-1504. doi: 10.1093/biolre/ioz023. Biol Reprod. 2019. PMID: 30770532 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007334/AI/NIAID NIH HHS/United States
- R56 AI020038/AI/NIAID NIH HHS/United States
- R37 AI020038/AI/NIAID NIH HHS/United States
- R01 AI097457/AI/NIAID NIH HHS/United States
- R01 AI020038/AI/NIAID NIH HHS/United States
- R01 AI065495/AI/NIAID NIH HHS/United States
- R01 AI113272/AI/NIAID NIH HHS/United States
- R21 AI117378/AI/NIAID NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- R56 AI108684/AI/NIAID NIH HHS/United States
- R01 EY016408/EY/NEI NIH HHS/United States
- R01 AI107020/AI/NIAID NIH HHS/United States
- P30 EY002162/EY/NEI NIH HHS/United States
- T32 EY007120/EY/NEI NIH HHS/United States
- U19 AI095261/AI/NIAID NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

