Trichostatin A Inhibits Epithelial Mesenchymal Transition Induced by TGF-β1 in Airway Epithelium
- PMID: 27571418
- PMCID: PMC5003433
- DOI: 10.1371/journal.pone.0162058
Trichostatin A Inhibits Epithelial Mesenchymal Transition Induced by TGF-β1 in Airway Epithelium
Abstract
Background and objectives: Tissue remodeling is believed to cause recalcitrant chronic rhinosinusitis (CRS). Epithelial-mesenchymal transition (EMT) is a novel clinical therapeutic target in many chronic airway diseases related with tissue remodeling. The aim of this study was to investigate the effect of trichostatin A (TSA) on transforming growth factor (TGF)-β1-induced EMT in airway epithelium and nasal tissue.
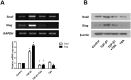
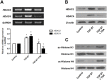
Materials and methods: A549 cells, primary nasal epithelial cells (PNECs), or inferior nasal turbinate organ culture were exposed to TSA prior to stimulation with TGF-β1. Expression levels of E-cadherin, vimentin, fibronectin, α-smooth muscle actin (SMA), histone deacetylase 2 (HDAC2), and HDAC4 were determined by western blotting and/or immunofluorescent staining. Hyperacetylation of histone H2 and H4 by TSA was measured by western blotting. After siHDAC transfection, the effects of HDAC2 and HDAC4 silencing on expression of E-cadherin, vimentin, fibronectin, α-SMA, HDAC2, and HDAC4 in TGF-β1-induced A549 were determined by RT-PCR and/or western blotting. We assessed the change in migration capacity of A549 cells by using cell migration assay and transwell invasion assay.
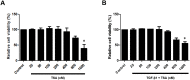
Results: TGF-β1 altered mRNA and protein expression levels of EMT markers including E-cadherin, vimentin, fibronectin, α-SMA, slug, and snail in A549 cells. Inhibition and silencing of HDAC2 and HDAC4 by TSA and siRNA enhanced TGF-β1-induced EMT in A549 cells. TSA blocked the effect of TGF-β1 on the migratory ability of A549 cells. In experiments using PNECs and inferior turbinate organ cultures, TSA suppressed expression of EMT markers induced by TGF-β1.
Conclusions: We showed that EMT is induced by TGF-β1 in airway epithelial cells and nasal tissue via activation of HDAC2 and HDAC4, and that inhibition of HDAC2 and HDAC4 by TSA reduces TGF-β1-induced EMT. This observation indicates that histone deacetylase inhibitors such as TSA could be potential candidates for treatment of recalcitrant CRS related with tissue remodeling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Chemical Chaperone of Endoplasmic Reticulum Stress Inhibits Epithelial-Mesenchymal Transition Induced by TGF-β1 in Airway Epithelium via the c-Src Pathway.Mediators Inflamm. 2017;2017:8123281. doi: 10.1155/2017/8123281. Epub 2017 Jul 19. Mediators Inflamm. 2017. PMID: 28804222 Free PMC article.
-
Effects of histone deacetylase inhibitor on extracellular matrix production in human nasal polyp organ cultures.Am J Rhinol Allergy. 2013 Jan;27(1):18-23. doi: 10.2500/ajra.2013.27.3827. Am J Rhinol Allergy. 2013. PMID: 23406592
-
Epigenetic regulation of myofibroblast differentiation and extracellular matrix production in nasal polyp-derived fibroblasts.Clin Exp Allergy. 2012 Jun;42(6):872-82. doi: 10.1111/j.1365-2222.2011.03931.x. Clin Exp Allergy. 2012. PMID: 22239687
-
[Investigation of Drug-induced Lung Injury for the Development of a Novel Therapeutic Approach].Yakugaku Zasshi. 2020;140(1):15-22. doi: 10.1248/yakushi.19-00133. Yakugaku Zasshi. 2020. PMID: 31902879 Review. Japanese.
-
Epithelial-mesenchymal transition in the lacrimal gland morphogenesis, damage and repair.Ocul Surf. 2023 Jul;29:401-405. doi: 10.1016/j.jtos.2023.06.008. Epub 2023 Jun 14. Ocul Surf. 2023. PMID: 37321448 Review.
Cited by
-
Panax Notoginseng Saponins Regulate Transforming Growth Factor-β1 through MAPK and Snail/TWIST1 Signaling Pathway to Inhibit Epithelial-Mesenchymal Transition of Pulmonary Fibrosis in A549 Cells.Evid Based Complement Alternat Med. 2022 Jul 12;2022:3744618. doi: 10.1155/2022/3744618. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35865337 Free PMC article.
-
Chemical Chaperone of Endoplasmic Reticulum Stress Inhibits Epithelial-Mesenchymal Transition Induced by TGF-β1 in Airway Epithelium via the c-Src Pathway.Mediators Inflamm. 2017;2017:8123281. doi: 10.1155/2017/8123281. Epub 2017 Jul 19. Mediators Inflamm. 2017. PMID: 28804222 Free PMC article.
-
Epithelial-Mesenchymal Transition in Chronic Rhinosinusitis.J Rhinol. 2024 Jul;31(2):67-77. doi: 10.18787/jr.2024.00022. Epub 2024 Jul 31. J Rhinol. 2024. PMID: 39664411 Free PMC article. Review.
-
Pharmacological Properties of Trichostatin A, Focusing on the Anticancer Potential: A Comprehensive Review.Pharmaceuticals (Basel). 2022 Oct 8;15(10):1235. doi: 10.3390/ph15101235. Pharmaceuticals (Basel). 2022. PMID: 36297347 Free PMC article. Review.
-
Approaches in Hydroxytyrosol Supplementation on Epithelial-Mesenchymal Transition in TGFβ1-Induced Human Respiratory Epithelial Cells.Int J Mol Sci. 2023 Feb 16;24(4):3974. doi: 10.3390/ijms24043974. Int J Mol Sci. 2023. PMID: 36835384 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

