'Trial Exegesis': Methods for Synthesizing Clinical and Patient Reported Outcome (PRO) Data in Trials to Inform Clinical Practice. A Systematic Review
- PMID: 27571514
- PMCID: PMC5003376
- DOI: 10.1371/journal.pone.0160998
'Trial Exegesis': Methods for Synthesizing Clinical and Patient Reported Outcome (PRO) Data in Trials to Inform Clinical Practice. A Systematic Review
Abstract
Purpose: The CONSORT extension for patient reported outcomes (PROs) aims to improve reporting, but guidance on the optimal integration with clinical data is lacking. This study examines in detail the reporting of PROs and clinical data from randomized controlled trials (RCTs) in gastro-intestinal cancer to inform design and reporting of combined PRO and clinical data from trials to improve the 'take home' message for clinicians to use in practice.
Materials and methods: The case study was undertaken in gastro-intestinal cancer trials. Well-conducted RCTs reporting PROs with validated instruments were identified and categorized into those combining PRO and clinical data in a single paper, or those separating data into linked primary and supplemental papers. Qualitative methods were developed to examine reporting of the critical interpretation of the trial results (trial exegesis) in the papers in relation of the PRO and clinical outcomes and applied to each publication category. Results were used to inform recommendations for practice.
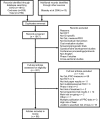
Results: From 1917 screened abstracts, 49 high quality RCTs were identified reported in 36 combined and 15 linked primary and supplemental papers. In-depth analysis of manuscript text identified three categories for understanding trial exegesis: where authors reported a "detailed", "general", or absent PRO rationale and integrated interpretation of clinical and PRO results. A total of 11 (30%) and 6 (16%) combined papers reported "detailed" PRO rationale and integrated interpretation of results although only 2 (14%) and 1 (7%) primary papers achieved the same standard respectively. Supplemental papers provide better information with 11 (73%) and 3 (20%) achieving "detailed" rationale and integrated interpretation of results. Supplemental papers, however, were published a median of 20 months after the primary RCT data in lower impact factor journals (median 16.8 versus 5.2).
Conclusion: It is recommended that single papers, with detailed PRO rationale and integrated PRO and clinical data are published to optimize trial exegesis. Further work to examine whether this improves the use of PRO data to inform practice is needed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.Health Technol Assess. 2004 Sep;8(36):iii-iv, ix-xi, 1-158. doi: 10.3310/hta8360. Health Technol Assess. 2004. PMID: 15361314
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
Cited by
-
The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis.Health Qual Life Outcomes. 2019 Oct 16;17(1):156. doi: 10.1186/s12955-019-1220-z. Health Qual Life Outcomes. 2019. PMID: 31619266 Free PMC article.
-
How patient-generated health data and patient-reported outcomes affect patient-clinician relationships: A systematic review.Health Informatics J. 2020 Dec;26(4):2689-2706. doi: 10.1177/1460458220928184. Epub 2020 Jun 20. Health Informatics J. 2020. PMID: 32567460 Free PMC article.
-
Outcome selection, measurement and reporting for new surgical procedures and devices: a systematic review of IDEAL/IDEAL-D studies to inform development of a core outcome set.BJS Open. 2020 Oct 4;4(6):1072-83. doi: 10.1002/bjs5.50358. Online ahead of print. BJS Open. 2020. PMID: 33016009 Free PMC article. Review.
References
-
- Blazeby JM, Avery K, Sprangers M, Pikhart H, Fayers P, Donovan J. Health-related quality of life measurement in randomized clinical trials in surgical oncology. Journal of Clinical Oncology. 2006;24(19):3178–86. - PubMed
-
- The Cochrane C. Cochrane handbook for systematic reviews of interventions2009.
-
- Au HJ, Karapetis CS, O'Callaghan CJ, Tu D, Moore MJ, Zalcberg JR, et al. Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: overall and KRAS-specific results of the NCIC CTG and AGITG CO.17 Trial. Journal of Clinical Oncology. 2009;27(11):1822–8. 10.1200/JCO.2008.19.6048 - DOI - PubMed
-
- King PM, Blazeby JM, Ewings P, Franks PJ, Longman RJ, Kendrick AH, et al. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. British Journal of Surgery. 2006;93(3):300–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous