Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin
- PMID: 27571886
- PMCID: PMC5464255
- DOI: 10.1093/neuonc/now174
Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin
Abstract
Background: Deprivation of tumor bioenergetics by inhibition of multiple energy pathways has been suggested as an effective therapeutic approach for various human tumors. However, this idea has not been evaluated in glioblastoma (GBM). We hypothesized that dual inhibition of glycolysis and oxidative phosphorylation could effectively suppress GBM tumorspheres (TS).
Methods: Effects of 2-deoxyglucose (2DG) and metformin, alone and in combination, on GBM-TS were evaluated. Viability, cellular energy metabolism status, stemness, invasive properties, and GBM-TS transcriptomes were examined. In vivo efficacy was tested in a mouse orthotopic xenograft model.
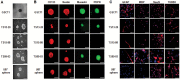
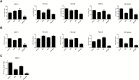
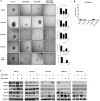
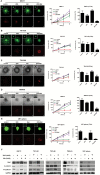
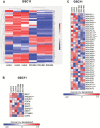
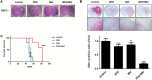
Results: GBM-TS viability was decreased by the combination of 2DG and metformin. ATP assay and PET showed that cellular energy metabolism was also decreased by this combination. Sphere formation, expression of stemness-related proteins, and invasive capacity of GBM-TS were also significantly suppressed by combined treatment with 2DG and metformin. A transcriptome analysis showed that the expression levels of stemness- and epithelial mesenchymal transition-related genes were also significantly downregulated by combination of 2DG and metformin. Combination treatment also prolonged survival of tumor-bearing mice and decreased invasiveness of GBM-TS.
Conclusion: The combination of 2DG and metformin effectively decreased the stemness and invasive properties of GBM-TS and showed a potential survival benefit in a mouse orthotopic xenograft model. Our findings suggest that targeting TS-forming cells by this dual inhibition of cellular bioenergetics warrants expedited clinical evaluation for the treatment of GBM.
Keywords: 2-deoxyglucose; glioblastoma; invasion; metformin; stemness.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures
References
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. - PubMed
-
- Binda E, Reynolds BA, Vescovi AL. Glioma stem cells: turpis omen in nomen? (The evil in the name?). J Intern Med. 2014;276(1):25–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

