The microenvironment of injured murine gut elicits a local pro-restitutive microbiota
- PMID: 27571978
- PMCID: PMC5076466
- DOI: 10.1038/nmicrobiol.2015.21
The microenvironment of injured murine gut elicits a local pro-restitutive microbiota
Abstract
The mammalian intestine houses a complex microbial community, which influences normal epithelial growth and development, and is integral to the repair of damaged intestinal mucosa(1-3). Restitution of injured mucosa involves the recruitment of immune cells, epithelial migration and proliferation(4,5). Although microenvironmental alterations have been described in wound healing(6), a role for extrinsic influences, such as members of the microbiota, has not been reported. Here, we show that a distinct subpopulation of the normal mucosal-associated gut microbiota expands and preferentially colonizes sites of damaged murine mucosa in response to local environmental cues. Our results demonstrate that formyl peptide receptor 1 (FPR1) and neutrophilic NADPH oxidase (NOX2) are required for the rapid depletion of microenvironmental oxygen and compensatory responses, resulting in a dramatic enrichment of an anaerobic bacterial consortium. Furthermore, the dominant member of this wound-mucosa-associated microbiota, Akkermansia muciniphila (an anaerobic, mucinophilic gut symbiont(7,8)), stimulated proliferation and migration of enterocytes adjacent to the colonic wounds in a process involving FPR1 and intestinal epithelial-cell-specific NOX1-dependent redox signalling. These findings thus demonstrate how wound microenvironments induce the rapid emergence of 'probiont' species that contribute to enhanced repair of mucosal wounds. Such microorganisms could be exploited as potential therapeutics.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
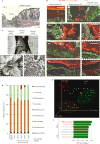
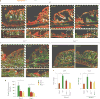
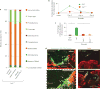
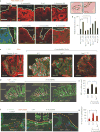
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

