Mapping rare, deleterious mutations in Factor H: Association with early onset, drusen burden, and lower antigenic levels in familial AMD
- PMID: 27572114
- PMCID: PMC5004131
- DOI: 10.1038/srep31531
Mapping rare, deleterious mutations in Factor H: Association with early onset, drusen burden, and lower antigenic levels in familial AMD
Abstract
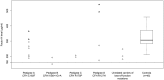
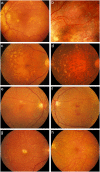
The genetic architecture of age-related macular degeneration (AMD) involves numerous genetic variants, both common and rare, in the coding region of complement factor H (CFH). While these variants explain high disease burden in some families, they fail to explain the pathology in all. We selected families whose AMD was unexplained by known variants and performed whole exome sequencing to probe for other rare, highly penetrant variants. We identified four rare loss-of-function variants in CFH associated with AMD. Missense variant CFH 1:196646753 (C192F) segregated perfectly within a family characterized by advanced AMD and drusen temporal to the macula. Two families, each comprising a pair of affected siblings with extensive extramacular drusen, carried essential splice site variant CFH 1:196648924 (IVS6+1G>A) or missense variant rs139360826 (R175P). In a fourth family, missense variant rs121913058 (R127H) was associated with AMD. Most carriers had early onset bilateral advanced AMD and extramacular drusen. Carriers tended to have low serum Factor H levels, especially carriers of the splice variant. One missense variant (R127H) has been previously shown not to be secreted. The two other missense variants were produced recombinantly: compared to wild type, one (R175P) had no functional activity and the other (C192F) had decreased secretion.
Figures

References
-
- Weeks D. E. et al.. Age-related maculopathy: an expanded genome-wide scan with evidence of susceptibility loci within the 1q31 and 17q25 regions. Am. J. Ophthalmol. 132, 682–692 (2001). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

