Pharmacokinetics and analgesic effects of methadone in children and adults with sickle cell disease
- PMID: 27572136
- PMCID: PMC5411015
- DOI: 10.1002/pbc.26207
Pharmacokinetics and analgesic effects of methadone in children and adults with sickle cell disease
Abstract
Background: Vaso-occlusive episodes (VOEs) are a significant source of morbidity among children and adults with sickle cell disease (SCD). There is little information on methadone use for SCD pain. This investigation evaluated methadone pharmacokinetics in children and adults with SCD, with a secondary aim to assess pain relief and opioid consumption.
Procedure: Participants included children (<18 years) and adults with a VOE requiring hospitalization. Patients were randomly assigned to receive standard care (opioid patient-controlled analgesia; control group) or one dose of intravenous methadone (0.1-0.125 mg/kg) in addition to standard care (methadone group). Venous methadone and metabolite concentrations were measured. Pain scores, pain relief scores, and opioid consumption were recorded.
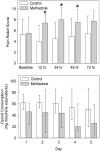
Results: Twenty-four children (12 methadone, 12 controls) and 23 adults (11 methadone, 12 controls) were studied. In children, the half-life of R- and S-methadone enantiomers was 34 ± 16 and 24 ± 9 hr, respectively. In adults, R- and S-methadone half-lives were 52 ± 17 and 38 ± 12 hr, respectively. Pain scores were lower (P = 0.002) and pain relief scores were higher (P = 0.0396) in children receiving methadone versus controls. There was no difference in pain scores and pain relief in adults receiving methadone versus controls. There was no difference in opioid consumption between methadone and control groups, in both adults and children.
Conclusions: Intravenous methadone disposition in children and adults with SCD was comparable to that in subjects without SCD from prior studies. Methadone produced more pain relief than standard care in children with SCD. Higher methadone doses may be more effective and should be evaluated in both children and adults with SCD.
Trial registration: ClinicalTrials.gov NCT00761085.
Keywords: analgesics; methadone; pediatric; sickle cell disease.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest: No author has any conflict of interest
Figures



Comment in
-
Comment on: Pharmacokinetics and analgesic effects of methadone in children and adults with sickle cell disease.Pediatr Blood Cancer. 2017 May;64(5). doi: 10.1002/pbc.26307. Epub 2016 Oct 26. Pediatr Blood Cancer. 2017. PMID: 27781384 No abstract available.
-
Reply to comment on: Pharmacokinetics and analgesic effects of methadone in children and adults with sickle cell disease.Pediatr Blood Cancer. 2017 May;64(5). doi: 10.1002/pbc.26343. Epub 2016 Nov 10. Pediatr Blood Cancer. 2017. PMID: 27860157 No abstract available.
References
-
- Platt OSBD, Rosse WF. Mortality in sickle cell disease: Life expectancy and risk factors for early death. NEJM. 1994;330:1639–1644. - PubMed
-
- Shapiro BS, Dinges DF, Carota Orne E, et al. Home management of sickle cell-related pain in children and adolescents: a natural history and impact on school attendance. Pain. 1995;61:139–144. - PubMed
-
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. N Engl J Med. 1991;325(1):11–15. - PubMed
-
- Panepinto J, Brousseau D, Hillery C, Scott J. Variation in hospitalizations and hospital length of stay in children with vaso-occlusive crises in Sickle Cell Disease. Pediatr Blood Cancer. 2005;44:182–186. - PubMed
-
- Stearns SDSC, Carter BD. Psychological Ramifications of Pediatric Pain. Clin Ped Emerg Med. 2000;1:299–305.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

