Emtricitabine-Triphosphate in Dried Blood Spots as a Marker of Recent Dosing
- PMID: 27572401
- PMCID: PMC5075074
- DOI: 10.1128/AAC.01017-16
Emtricitabine-Triphosphate in Dried Blood Spots as a Marker of Recent Dosing
Abstract
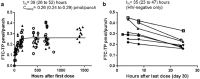
New objective measures of antiretroviral adherence are needed. We determined if emtricitabine triphosphate (FTC-TP) in dried blood spots (DBS) can be used as a marker of recent dosing with tenofovir disoproxil fumarate-emtricitabine (TDF-FTC). The half-life of FTC-TP was estimated in DBS samples obtained from an intensive pharmacokinetic (PK) study of coformulated TDF-FTC in HIV-negative and HIV-infected participants. The concordance of quantifiable FTC-TP in DBS with tenofovir (TFV)/FTC in plasma was evaluated by utilizing paired plasma-DBS samples from participants enrolled in 2 large preexposure prophylaxis (PrEP) open-label trials. The time to FTC-TP nondetectability after TDF-FTC dosing was evaluated utilizing DBS from HIV-negative participants enrolled in a directly observed therapy study of variable adherence to TDF-FTC. The mean (95% confidence interval [CI]) terminal half-life of FTC-TP in the PK study was 35 (23 to 47) h. A total of 143/163 (88%) samples obtained 0 to 48 h post-TDF-FTC dose had quantifiable FTC-TP in DBS, compared with 2/93 (2%) and 0/87 (0%) obtained >48 and >96 h postdose. In 746 paired plasma-DBS samples from 445 participants enrolled in PrEP trials, when both TFV/FTC in plasma were below the limit of quantification, FTC-TP was as well in 98.9% of the samples, and when either TFV or FTC in plasma was quantifiable, FTC-TP was as well in 90.5% of the samples. The half-life of FTC-TP in DBS is short relative to that of TFV-diphosphate (TFV-DP), making it a surrogate for TFV-FTC detection in plasma. FTC-TP can be quantified in DBS simultaneously with TFV-DP, which quantifies cumulative adherence to TDF-FTC. (The clinical trials discussed in this article have been registered at ClinicalTrials.gov under identifiers NCT01040091, NCT02022657, NCT00458393, NCT01772823, and NCT02012621.).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV, i PrEx Study Team. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi:10.1056/NEJMoa1011205. - DOI - PMC - PubMed
-
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C, Partners PrEP Study Team. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367:399–410. doi:10.1056/NEJMoa1108524. - DOI - PMC - PubMed
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT, TDF2 Study Group. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367:423–434. doi:10.1056/NEJMoa1110711. - DOI - PubMed
-
- Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, Chiamwongpaet S, Kitisin P, Natrujirote P, Kittimunkong S, Chuachoowong R, Gvetadze RJ, McNicholl JM, Paxton LA, Curlin ME, Hendrix CW, Vanichseni S, Bangkok Tenofovir Study Group. 2013. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 381:2083–2090. doi:10.1016/S0140-6736(13)61127-7. - DOI - PubMed
-
- Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, Hosek S, Mosquera C, Casapia M, Montoya O, Buchbinder S, Veloso VG, Mayer K, Chariyalertsak S, Bekker LG, Kallas EG, Schechter M, Guanira J, Bushman L, Burns DN, Rooney JF, Glidden DV, iPrEx Study Team. 2014. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 14:820–829. doi:10.1016/S1473-3099(14)70847-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

