Maternal colonization with Streptococcus agalactiae and associated stillbirth and neonatal disease in coastal Kenya
- PMID: 27572968
- PMCID: PMC4936517
- DOI: 10.1038/nmicrobiol.2016.67
Maternal colonization with Streptococcus agalactiae and associated stillbirth and neonatal disease in coastal Kenya
Abstract
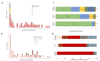
Streptococcus agalactiae (group B streptococcus, GBS) causes neonatal disease and stillbirth, but its burden in sub-Saharan Africa is uncertain. We assessed maternal recto-vaginal GBS colonization (7,967 women), stillbirth and neonatal disease. Whole-genome sequencing was used to determine serotypes, sequence types and phylogeny. We found low maternal GBS colonization prevalence (934/7,967, 12%), but comparatively high incidence of GBS-associated stillbirth and early onset neonatal disease (EOD) in hospital (0.91 (0.25-2.3)/1,000 births and 0.76 (0.25-1.77)/1,000 live births, respectively). However, using a population denominator, EOD incidence was considerably reduced (0.13 (0.07-0.21)/1,000 live births). Treated cases of EOD had very high case fatality (17/36, 47%), especially within 24 h of birth, making under-ascertainment of community-born cases highly likely, both here and in similar facility-based studies. Maternal GBS colonization was less common in women with low socio-economic status, HIV infection and undernutrition, but when GBS-colonized, they were more probably colonized by the most virulent clone, CC17. CC17 accounted for 267/915 (29%) of maternal colonizing (265/267 (99%) serotype III; 2/267 (0.7%) serotype IV) and 51/73 (70%) of neonatal disease cases (all serotype III). Trivalent (Ia/II/III) and pentavalent (Ia/Ib/II/III/V) vaccines would cover 71/73 (97%) and 72/73 (99%) of disease-causing serotypes, respectively. Serotype IV should be considered for inclusion, with evidence of capsular switching in CC17 strains.
Conflict of interest statement
We declare no competing interests.
Figures

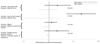

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

