Interrupting Biosynthesis of O Antigen or the Lipopolysaccharide Core Produces Morphological Defects in Escherichia coli by Sequestering Undecaprenyl Phosphate
- PMID: 27573014
- PMCID: PMC5075036
- DOI: 10.1128/JB.00550-16
Interrupting Biosynthesis of O Antigen or the Lipopolysaccharide Core Produces Morphological Defects in Escherichia coli by Sequestering Undecaprenyl Phosphate
Abstract
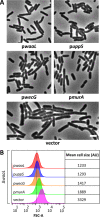
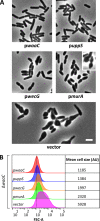
Undecaprenyl phosphate (Und-P) is a member of the family of essential polyprenyl phosphate lipid carriers and in the Gram-negative bacterium Escherichia coli is required for synthesizing the peptidoglycan (PG) cell wall, enterobacterial common antigen (ECA), O antigen, and colanic acid. Previously, we found that interruption of ECA biosynthesis indirectly alters PG synthesis by sequestering Und-P via dead-end intermediates, causing morphological defects. To determine if competition for Und-P was a more general phenomenon, we determined if O-antigen intermediates caused similar effects. Indeed, disrupting the synthesis of O antigen or the lipopolysaccharide core oligosaccharide induced cell shape deformities, which were suppressed by preventing the initiation of O-antigen biosynthesis or by manipulating Und-P metabolism. We conclude that accumulation of O-antigen intermediates alters PG synthesis by sequestering Und-P. Importantly, many previous experiments addressed the physiological functions of various oligosaccharides and glycoconjugates, but these studies employed mutants that accumulate deleterious intermediates. Thus, conclusions based on these experiments must be reevaluated to account for possible indirect effects of Und-P sequestration.
Importance: Bacteria use long-chain isoprenoids like undecaprenyl phosphate (Und-P) as lipid carriers to assemble numerous glycan polymers that comprise the cell envelope. In any one bacterium, multiple oligosaccharide biosynthetic pathways compete for a common pool of Und-P, which means that disruptions in one pathway may produce secondary consequences that affect the others. Using the Gram-negative bacterium Escherichia coli as a model, we demonstrate that interruption of the biogenesis of O antigen, a major outer membrane component, indirectly impairs peptidoglycan synthesis by sequestering Und-P into dead-end intermediates. These results strongly argue that the functions of many Und-P-utilizing pathways must be reevaluated, because much of our current understanding is based on experiments that did not control for these unintended secondary effects.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Dead-end intermediates in the enterobacterial common antigen pathway induce morphological defects in Escherichia coli by competing for undecaprenyl phosphate.Mol Microbiol. 2016 Apr;100(1):1-14. doi: 10.1111/mmi.13284. Epub 2015 Dec 22. Mol Microbiol. 2016. PMID: 26593043 Free PMC article.
-
A Defective Undecaprenyl Pyrophosphate Synthase Induces Growth and Morphological Defects That Are Suppressed by Mutations in the Isoprenoid Pathway of Escherichia coli.J Bacteriol. 2018 Aug 24;200(18):e00255-18. doi: 10.1128/JB.00255-18. Print 2018 Sep 15. J Bacteriol. 2018. PMID: 29986944 Free PMC article.
-
An Escherichia coli undecaprenyl-pyrophosphate phosphatase implicated in undecaprenyl phosphate recycling.Microbiology (Reading). 2007 Aug;153(Pt 8):2518-2529. doi: 10.1099/mic.0.2007/006312-0. Microbiology (Reading). 2007. PMID: 17660416
-
Common themes in glycoconjugate assembly using the biogenesis of O-antigen lipopolysaccharide as a model system.Biochemistry (Mosc). 2011 Jul;76(7):729-35. doi: 10.1134/S0006297911070029. Biochemistry (Mosc). 2011. PMID: 21999533 Review.
-
Deciphering the metabolism of undecaprenyl-phosphate: the bacterial cell-wall unit carrier at the membrane frontier.Microb Drug Resist. 2014 Jun;20(3):199-214. doi: 10.1089/mdr.2014.0035. Epub 2014 May 5. Microb Drug Resist. 2014. PMID: 24799078 Free PMC article. Review.
Cited by
-
Morphological and Transcriptional Responses to CRISPRi Knockdown of Essential Genes in Escherichia coli.mBio. 2021 Oct 26;12(5):e0256121. doi: 10.1128/mBio.02561-21. Epub 2021 Oct 12. mBio. 2021. PMID: 34634934 Free PMC article.
-
Quantitative Analyses Reveal Novel Roles for N-Glycosylation in a Major Enteric Bacterial Pathogen.mBio. 2019 Apr 23;10(2):e00297-19. doi: 10.1128/mBio.00297-19. mBio. 2019. PMID: 31015322 Free PMC article.
-
Genome-scale analysis of essential gene knockout mutants to identify an antibiotic target process.Antimicrob Agents Chemother. 2023 Dec 14;67(12):e0110223. doi: 10.1128/aac.01102-23. Epub 2023 Nov 15. Antimicrob Agents Chemother. 2023. PMID: 37966228 Free PMC article.
-
Diacylglycerol Kinase A Is Essential for Polymyxin Resistance Provided by EptA, MCR-1, and Other Lipid A Phosphoethanolamine Transferases.J Bacteriol. 2022 Feb 15;204(2):e0049821. doi: 10.1128/JB.00498-21. Epub 2021 Nov 29. J Bacteriol. 2022. PMID: 34843376 Free PMC article.
-
The conserved transcriptional regulator CdnL is required for metabolic homeostasis and morphogenesis in Caulobacter.PLoS Genet. 2020 Jan 21;16(1):e1008591. doi: 10.1371/journal.pgen.1008591. eCollection 2020 Jan. PLoS Genet. 2020. PMID: 31961855 Free PMC article.
References
-
- Grover S, Alderwick LJ, Mishra AK, Krumbach K, Marienhagen J, Eggeling L, Bhatt A, Besra GS. 2014. Benzothiazinones mediate killing of Corynebacterineae by blocking decaprenyl phosphate recycling involved in cell wall biosynthesis. J Biol Chem 289:6177–6187. doi:10.1074/jbc.M113.522623. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

