A-Disintegrin and Metalloproteinase (ADAM) 17 Enzymatically Degrades Interferon-gamma
- PMID: 27573075
- PMCID: PMC5004192
- DOI: 10.1038/srep32259
A-Disintegrin and Metalloproteinase (ADAM) 17 Enzymatically Degrades Interferon-gamma
Abstract
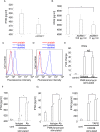
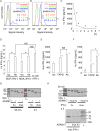
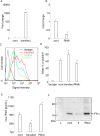
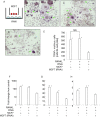
Interferon-gamma (IFN-γ) is a pleiotropic cytokine that exerts anti-tumor and anti-osteoclastogenic effects. Although transcriptional and post-transcriptional regulation of IFN-γ is well understood, subsequent modifications of secreted IFN-γ are not fully elucidated. Previous research indicates that some cancer cells escape immune surveillance and metastasize into bone tissue by inducing osteoclastic bone resorption. Peptidases of the a-disintegrin and metalloproteinase (ADAM) family are implicated in cancer cell proliferation and tumor progression. We hypothesized that the ADAM enzymes expressed by cancer cells degrades IFN-γ and attenuates IFN-γ-mediated anti-tumorigenic and anti-osteoclastogenic effects. Recombinant ADAM17 degraded IFN-γ into small fragments. The addition of ADAM17 to the culture supernatant of stimulated mouse splenocytes decreased IFN-γ concentration. However, ADAM17 inhibition in the stimulated mouse T-cells prevented IFN-γ degradation. ADAM17-expressing human breast cancer cell lines MCF-7 and MDA-MB-453 also degraded recombinant IFN-γ, but this was attenuated by ADAM17 inhibition. Degraded IFN-γ lost the functionality including the inhibititory effect on osteoclastogenesis. This is the first study to demonstrate the extracellular proteolytic degradation of IFN-γ by ADAM17. These results suggest that ADAM17-mediated degradation of IFN-γ may block the anti-tumorigenic and anti-osteoclastogenic effects of IFN-γ. ADAM17 inhibition may be useful for the treatment of attenuated cancer immune surveillance and/or bone metastases.
Figures


Similar articles
-
Effects of ADAM10 and ADAM17 Inhibitors on Natural Killer Cell Expansion and Antibody-dependent Cellular Cytotoxicity Against Breast Cancer Cells In Vitro.Anticancer Res. 2017 Oct;37(10):5507-5513. doi: 10.21873/anticanres.11981. Anticancer Res. 2017. PMID: 28982863
-
Interferon-gamma down-regulates expression of tumor necrosis factor-alpha converting enzyme/a disintegrin and metalloproteinase 17 in activated hepatic stellate cells of rats.Int J Mol Med. 2006 Apr;17(4):605-16. Int J Mol Med. 2006. PMID: 16525716
-
Epithelial Cell-Derived a Disintegrin and Metalloproteinase-17 Confers Resistance to Colonic Inflammation Through EGFR Activation.EBioMedicine. 2016 Feb 9;5:114-24. doi: 10.1016/j.ebiom.2016.02.007. eCollection 2016 Mar. EBioMedicine. 2016. PMID: 27077118 Free PMC article.
-
Molecular switch in human diseases-disintegrin and metalloproteinases, ADAM17.Aging (Albany NY). 2021 Jun 28;13(12):16859-16872. doi: 10.18632/aging.203200. Epub 2021 Jun 28. Aging (Albany NY). 2021. PMID: 34182543 Free PMC article. Review.
-
The shedding protease ADAM17: Physiology and pathophysiology.Biochim Biophys Acta Mol Cell Res. 2017 Nov;1864(11 Pt B):2059-2070. doi: 10.1016/j.bbamcr.2017.07.001. Epub 2017 Jul 11. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 28705384 Review.
Cited by
-
SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications.J Mol Cell Cardiol. 2020 Jul;144:47-53. doi: 10.1016/j.yjmcc.2020.04.031. Epub 2020 Apr 30. J Mol Cell Cardiol. 2020. PMID: 32360703 Free PMC article. Review.
-
Anti-ADAM17 monoclonal antibody MEDI3622 increases IFNγ production by human NK cells in the presence of antibody-bound tumor cells.Cancer Immunol Immunother. 2018 Sep;67(9):1407-1416. doi: 10.1007/s00262-018-2193-1. Epub 2018 Jul 5. Cancer Immunol Immunother. 2018. PMID: 29978334 Free PMC article.
-
Immunomodulatory role of metalloproteinase ADAM17 in tumor development.Front Immunol. 2022 Nov 17;13:1059376. doi: 10.3389/fimmu.2022.1059376. eCollection 2022. Front Immunol. 2022. PMID: 36466812 Free PMC article. Review.
-
TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status.J Allergy Clin Immunol. 2018 Jun;141(6):2048-2060.e13. doi: 10.1016/j.jaci.2017.08.020. Epub 2017 Sep 20. J Allergy Clin Immunol. 2018. PMID: 28939412 Free PMC article.
-
The role of myeloid derived suppressor cells in musculoskeletal disorders.Front Immunol. 2023 Mar 3;14:1139683. doi: 10.3389/fimmu.2023.1139683. eCollection 2023. Front Immunol. 2023. PMID: 36936946 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

