Phenotypic lentivirus screens to identify functional single domain antibodies
- PMID: 27573105
- PMCID: PMC5010022
- DOI: 10.1038/nmicrobiol.2016.80
Phenotypic lentivirus screens to identify functional single domain antibodies
Abstract
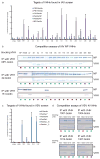
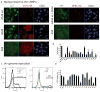
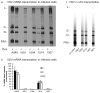
Manipulation of proteins is key in assessing their in vivo function. Although genetic ablation is straightforward, reversible and specific perturbation of protein function remains a challenge. Single domain antibody fragments, such as camelid-derived VHHs, can serve as inhibitors or activators of intracellular protein function, but functional testing of identified VHHs is laborious. To address this challenge, we have developed a lentiviral screening approach to identify VHHs that elicit a phenotype when expressed intracellularly. We identified 19 antiviral VHHs that protect human A549 cells from lethal infection with influenza A virus (IAV) or vesicular stomatitis virus (VSV), respectively. Both negative-sense RNA viruses are vulnerable to VHHs uniquely specific for their respective nucleoproteins. Antiviral VHHs prevented nuclear import of viral ribonucleoproteins or mRNA transcription, respectively, and may provide clues for novel antiviral reagents. In principle, the screening approach described here should be applicable to identify inhibitors of any pathogen or biological pathway.
Figures



Similar articles
-
Phenotypic Lentivirus Screens to Identify Antiviral Single Domain Antibodies.Methods Mol Biol. 2018;1836:139-158. doi: 10.1007/978-1-4939-8678-1_7. Methods Mol Biol. 2018. PMID: 30151572
-
Intracellular expression of camelid single-domain antibodies specific for influenza virus nucleoprotein uncovers distinct features of its nuclear localization.J Virol. 2015 Mar;89(5):2792-800. doi: 10.1128/JVI.02693-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540369 Free PMC article.
-
The Antiviral Mechanism of an Influenza A Virus Nucleoprotein-Specific Single-Domain Antibody Fragment.mBio. 2016 Dec 13;7(6):e01569-16. doi: 10.1128/mBio.01569-16. mBio. 2016. PMID: 27965447 Free PMC article.
-
Application of camelid heavy-chain variable domains (VHHs) in prevention and treatment of bacterial and viral infections.Int Rev Immunol. 2018 Jan 2;37(1):69-76. doi: 10.1080/08830185.2017.1397657. Epub 2017 Nov 28. Int Rev Immunol. 2018. PMID: 29182399 Review.
-
Influenza virus-host interactomes as a basis for antiviral drug development.Curr Opin Virol. 2015 Oct;14:71-8. doi: 10.1016/j.coviro.2015.08.008. Epub 2015 Sep 13. Curr Opin Virol. 2015. PMID: 26364134 Free PMC article. Review.
Cited by
-
Antibodies and venom peptides: new modalities for ion channels.Nat Rev Drug Discov. 2019 May;18(5):339-357. doi: 10.1038/s41573-019-0013-8. Nat Rev Drug Discov. 2019. PMID: 30728472 Free PMC article. Review.
-
Antibody Libraries as Tools to Discover Functional Antibodies and Receptor Pleiotropism.Int J Mol Sci. 2021 Apr 16;22(8):4123. doi: 10.3390/ijms22084123. Int J Mol Sci. 2021. PMID: 33923551 Free PMC article. Review.
-
Exploring cellular biochemistry with nanobodies.J Biol Chem. 2020 Nov 6;295(45):15307-15327. doi: 10.1074/jbc.REV120.012960. Epub 2020 Aug 31. J Biol Chem. 2020. PMID: 32868455 Free PMC article. Review.
-
Multivariate mining of an alpaca immune repertoire identifies potent cross-neutralizing SARS-CoV-2 nanobodies.Sci Adv. 2022 Mar 25;8(12):eabm0220. doi: 10.1126/sciadv.abm0220. Epub 2022 Mar 25. Sci Adv. 2022. PMID: 35333580 Free PMC article.
-
Nanobody-based sandwich reporter system for living cell sensing influenza A virus infection.Sci Rep. 2019 Nov 4;9(1):15899. doi: 10.1038/s41598-019-52258-7. Sci Rep. 2019. PMID: 31685871 Free PMC article.
References
-
- Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nature reviews Genetics. 2014;15:321–334. - PubMed
-
- Cohen P. Guidelines for the effective use of chemical inhibitors of protein function to understand their roles in cell regulation. Biochem J. 2010;425:53–54. - PubMed
-
- Doxsey SJ, Brodsky FM, Blank GS, Helenius A. Inhibition of endocytosis by anti-clathrin antibodies. Cell. 1987;50:453–463. - PubMed
-
- Gargano N, Cattaneo A. Rescue of a neutralizing anti-viral antibody fragment from an intracellular polyclonal repertoire expressed in mammalian cells. FEBS Lett. 1997;414:537–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

