The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia
- PMID: 27573114
- PMCID: PMC5010011
- DOI: 10.1038/nmicrobiol.2016.108
The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia
Abstract
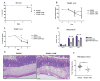
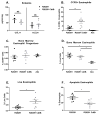
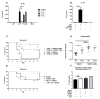
Clostridium difficile is the most common hospital acquired pathogen in the USA, and infection is, in many cases, fatal. Toxins A and B are its major virulence factors, but expression of a third toxin, known as C. difficile transferase (CDT), is increasingly common. An adenosine diphosphate (ADP)-ribosyltransferase that causes actin cytoskeletal disruption, CDT is typically produced by the major, hypervirulent strains and has been associated with more severe disease. Here, we show that CDT enhances the virulence of two PCR-ribotype 027 strains in mice. The toxin induces pathogenic host inflammation via a Toll-like receptor 2 (TLR2)-dependent pathway, resulting in the suppression of a protective host eosinophilic response. Finally, we show that restoration of TLR2-deficient eosinophils is sufficient for protection from a strain producing CDT. These findings offer an explanation for the enhanced virulence of CDT-expressing C. difficile and demonstrate a mechanism by which this binary toxin subverts the host immune response.
Figures
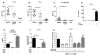

Similar articles
-
Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance.Gut Microbes. 2014 Jan-Feb;5(1):15-27. doi: 10.4161/gmic.26854. Epub 2013 Oct 31. Gut Microbes. 2014. PMID: 24253566 Free PMC article. Review.
-
Purified CDT toxins and a clean deletion within the CDT locus provide novel insights into the contribution of binary toxin in cellular inflammation and Clostridioides difficile infection.PLoS Pathog. 2024 Sep 19;20(9):e1012568. doi: 10.1371/journal.ppat.1012568. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39298531 Free PMC article.
-
Importance of toxin A, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain.J Infect Dis. 2014 Jan 1;209(1):83-6. doi: 10.1093/infdis/jit426. Epub 2013 Aug 9. J Infect Dis. 2014. PMID: 23935202 Free PMC article.
-
Binary Clostridium difficile toxin (CDT) - A virulence factor disturbing the cytoskeleton.Anaerobe. 2018 Oct;53:21-29. doi: 10.1016/j.anaerobe.2018.03.001. Epub 2018 Mar 7. Anaerobe. 2018. PMID: 29524654 Review.
-
Human intestinal enteroids as a model of Clostridioides difficile-induced enteritis.Am J Physiol Gastrointest Liver Physiol. 2020 May 1;318(5):G870-G888. doi: 10.1152/ajpgi.00045.2020. Epub 2020 Mar 30. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32223302 Free PMC article.
Cited by
-
Identification of TFPI as a receptor reveals recombination-driven receptor switching in Clostridioides difficile toxin B variants.Nat Commun. 2022 Nov 9;13(1):6786. doi: 10.1038/s41467-022-33964-9. Nat Commun. 2022. PMID: 36351897 Free PMC article.
-
A Prediction Model Incorporating Peripheral Eosinopenia as a Novel Risk Factor for Death After Hospitalization for Clostridioides difficile Infection.Gastro Hep Adv. 2022;1(1):38-44. doi: 10.1016/j.gastha.2021.10.002. Epub 2022 Feb 7. Gastro Hep Adv. 2022. PMID: 35974881 Free PMC article.
-
Defining the black box: a narrative review of factors associated with adverse outcomes from severe Clostridioides difficile infection.Therap Adv Gastroenterol. 2021 Oct 8;14:17562848211048127. doi: 10.1177/17562848211048127. eCollection 2021. Therap Adv Gastroenterol. 2021. PMID: 34646358 Free PMC article. Review.
-
Modulation of innate lymphoid cells by enteric bacterial pathogens.Front Immunol. 2023 Jul 6;14:1219072. doi: 10.3389/fimmu.2023.1219072. eCollection 2023. Front Immunol. 2023. PMID: 37483638 Free PMC article. Review.
-
Neutralization of macrophage migration inhibitory factor improves host survival after Clostridium difficile infection.Anaerobe. 2018 Oct;53:56-63. doi: 10.1016/j.anaerobe.2018.06.014. Epub 2018 Jun 23. Anaerobe. 2018. PMID: 29944928 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

