Regulation of Human Endonuclease V Activity and Relocalization to Cytoplasmic Stress Granules
- PMID: 27573237
- PMCID: PMC5076846
- DOI: 10.1074/jbc.M116.730911
Regulation of Human Endonuclease V Activity and Relocalization to Cytoplasmic Stress Granules
Abstract
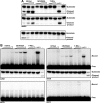
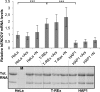
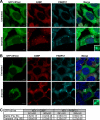
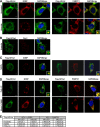
Endonuclease V (EndoV) is an enzyme with specificity for inosines in nucleic acids. Whereas the bacterial homologs are active on both DNA and RNA, the mammalian variants only cleave RNA, at least when assayed with recombinant proteins. Here we show that ectopically expressed, as well as endogenously expressed human (h)EndoV, share the same enzymatic properties as the recombinant protein and cleaves RNA with inosine but not DNA. In search for proteins interacting with hEndoV, polyadenylate-binding protein C1 (PABPC1) was identified. The association between PABPC1 and hEndoV is RNA dependent and furthermore, PABPC1 stimulates hEndoV activity and affinity for inosine-containing RNA. Upon cellular stress, PABPC1 relocates to cytoplasmic stress granules that are multimolecular aggregates of stalled translation initiation complexes formed to aid cell recovery. Arsenite, as well as other agents, triggered relocalization also of hEndoV to cytoplasmic stress granules. As inosines in RNA are highly abundant, hEndoV activity is likely regulated in cells to avoid aberrant cleavage of inosine-containing transcripts. Indeed, we find that hEndoV cleavage is inhibited by normal intracellular ATP concentrations. The ATP stores inside a cell do not overlay stress granules and we suggest that hEndoV is redistributed to stress granules as a strategy to create a local environment low in ATP to permit hEndoV activity.
Keywords: ATP; PABPC1; RNA; RNA processing; deamination; endonuclease V; inosine; nitrosative stress; ribonuclease; stress granule.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Lindahl T. (1993) Instability and decay of the primary structure of DNA. Nature 362, 709–715 - PubMed
-
- Pang B., McFaline J. L., Burgis N. E., Dong M., Taghizadeh K., Sullivan M. R., Elmquist C. E., Cunningham R. P., and Dedon P. C. (2012) Defects in purine nucleotide metabolism lead to substantial incorporation of xanthine and hypoxanthine into DNA and RNA. Proc. Natl. Acad. Sci. U.S.A. 109, 2319–2324 - PMC - PubMed
-
- Yasui M., Suenaga E., Koyama N., Masutani C., Hanaoka F., Gruz P.,Shibutani S., Nohmi T., Hayashi M., and Honma M. (2008) Miscoding properties of 2′-deoxyinosine, a nitric oxide-derived DNA adduct, during translesion synthesis catalyzed by human DNA polymerases. J. Mol. Biol. 377, 1015–1023 - PubMed
-
- Alseth I., Dalhus B., and Bjørås M. (2014) Inosine in DNA and RNA. Curr. Opin. Genet. Dev. 26, 116–123 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

