Ruthenium-Catalyzed Transfer Hydrogenation for C-C Bond Formation: Hydrohydroxyalkylation and Hydroaminoalkylation via Reactant Redox Pairs
- PMID: 27573275
- PMCID: PMC5099607
- DOI: 10.1007/s41061-016-0028-0
Ruthenium-Catalyzed Transfer Hydrogenation for C-C Bond Formation: Hydrohydroxyalkylation and Hydroaminoalkylation via Reactant Redox Pairs
Abstract
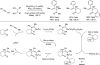
Merging the chemistry of transfer hydrogenation and carbonyl or imine addition, a broad new family of redox-neutral or reductive hydrohydroxyalkylations and hydroaminomethylations have been developed. In these processes, hydrogen redistribution between alcohols and π-unsaturated reactants is accompanied by C-C bond formation, enabling direct conversion of lower alcohols to higher alcohols. Similarly, hydrogen redistribution between amines to π-unsaturated reactants results in direct conversion of lower amines to higher amines. Alternatively, equivalent products of hydrohydroxyalkylation and hydroaminomethylation may be generated through the reaction of carbonyl compounds or imines with π-unsaturated reactants under the conditions of 2-propanol-mediated reductive coupling. Finally, using vicinally dioxygenated reactants, that is, diol, ketols, or diones, successive transfer hydrogenative coupling occurs to generate 2 C-C bonds, resulting in products of formal [4+2] cycloaddition.
Keywords: Borrowing Hydrogen; C-C Bond Formation; Enantioselective; Ruthenium; Transfer Hydrogenation.
Figures
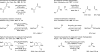





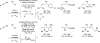
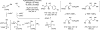
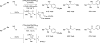









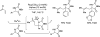
Similar articles
-
Ruthenium(0)-Catalyzed Cycloaddition of 1,2-Diols, Ketols, or Diones via Alcohol-Mediated Hydrogen Transfer.Angew Chem Int Ed Engl. 2018 Mar 12;57(12):3012-3021. doi: 10.1002/anie.201709916. Epub 2018 Feb 6. Angew Chem Int Ed Engl. 2018. PMID: 29068505 Free PMC article. Review.
-
Regioselective Hydrohydroxyalkylation of Styrene with Primary Alcohols or Aldehydes via Ruthenium-Catalyzed C-C Bond Forming Transfer Hydrogenation.Angew Chem Int Ed Engl. 2016 Dec 23;55(52):16119-16122. doi: 10.1002/anie.201609056. Epub 2016 Dec 2. Angew Chem Int Ed Engl. 2016. PMID: 27910228 Free PMC article.
-
Catalytic enantioselective C-H functionalization of alcohols by redox-triggered carbonyl addition: borrowing hydrogen, returning carbon.Angew Chem Int Ed Engl. 2014 Aug 25;53(35):9142-50. doi: 10.1002/anie.201403873. Epub 2014 Jul 23. Angew Chem Int Ed Engl. 2014. PMID: 25056771 Free PMC article. Review.
-
Ruthenium catalyzed C-C bond formation via transfer hydrogenation: branch-selective reductive coupling of allenes to paraformaldehyde and higher aldehydes.Org Lett. 2008 Jul 3;10(13):2705-8. doi: 10.1021/ol800836v. Epub 2008 Jun 6. Org Lett. 2008. PMID: 18533665 Free PMC article.
-
Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier.Acc Chem Res. 2017 Sep 19;50(9):2371-2380. doi: 10.1021/acs.accounts.7b00308. Epub 2017 Aug 9. Acc Chem Res. 2017. PMID: 28792731 Free PMC article.
Cited by
-
Iridium-, Ruthenium-, and Nickel-Catalyzed C-C Couplings of Methanol, Formaldehyde, and Ethanol with π-Unsaturated Pronucleophiles via Hydrogen Transfer.J Org Chem. 2023 Apr 21;88(8):4965-4974. doi: 10.1021/acs.joc.2c02356. Epub 2022 Nov 30. J Org Chem. 2023. PMID: 36449710 Free PMC article. Review.
-
Oxetane-, Azetidine-, and Bicyclopentane-Bearing N-Heterocycles from Ynones: Scaffold Diversification via Ruthenium-Catalyzed Oxidative Alkynylation.Org Lett. 2023 Aug 11;25(31):5907-5910. doi: 10.1021/acs.orglett.3c02213. Epub 2023 Aug 1. Org Lett. 2023. PMID: 37527501 Free PMC article.
-
Reductive C-C Coupling via Hydrogenation and Transfer Hydrogenation: Departure from Stoichiometric Metals in Carbonyl Addition.Curr Opin Green Sustain Chem. 2017 Oct;7:1-5. doi: 10.1016/j.cogsc.2017.06.006. Epub 2017 Jun 9. Curr Opin Green Sustain Chem. 2017. PMID: 29726550 Free PMC article.
-
Ruthenium(ii)-catalyzed olefination via carbonyl reductive cross-coupling.Chem Sci. 2017 Dec 1;8(12):8193-8197. doi: 10.1039/c7sc04207h. Epub 2017 Oct 9. Chem Sci. 2017. PMID: 29568466 Free PMC article.
-
Enantioselective Metal-Catalyzed Reductive Coupling of Alkynes with Carbonyl Compounds and Imines: Convergent Construction of Allylic Alcohols and Amines.ACS Catal. 2022 Jul 15;12(14):8164-8174. doi: 10.1021/acscatal.2c02444. Epub 2022 Jun 23. ACS Catal. 2022. PMID: 37082110 Free PMC article.
References
-
- Boyce J. Process of producing an edible compound. US1061254 A. 1913 May 6; James F. Boyce is credited with the hydrogenation of vegetable oils prior to Sabatier’s seminal work. To our knowledge, the earliest written record of Boyce’s work is a patent filed in 1911.
-
- Sabatier P, Senderens J-B. Action du Nickel sur l’Éthyléne. Synthéses de l’Éthane. C R Acad Sci Paris. 1897;124:1358–1360.
-
- Kagan HB. Victor Grignard and Paul Sabatier: Two Showcase Laureates of the Nobel Prize for Chemistry. Angew Chem Int Ed. 2012;51:7376–7382. - PubMed
-
- Voorhees V, Adams R. The Use of the Oxides of Platinum for the Catalytic Reduction of Organic Compounds. J Am Chem Soc. 1922;44:1397–1405.
-
- Calvin M, Polyani M. Homogeneous Catalytic Hydrogenation. Trans Faraday Soc. 1938;34:1181–1191.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
