Evaluating New Chemistry to Drive Molecular Discovery: Fit for Purpose?
- PMID: 27573303
- PMCID: PMC5113762
- DOI: 10.1002/anie.201604193
Evaluating New Chemistry to Drive Molecular Discovery: Fit for Purpose?
Abstract
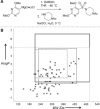
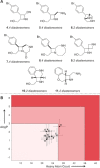
As our understanding of the impact of specific molecular properties on applications in discovery-based disciplines improves, the extent to which published synthetic methods meet (or do not meet) desirable criteria is ever clearer. Herein, we show how the application of simple (and in many cases freely available) computational tools can be used to develop a semiquantitative understanding of the potential of new methods to support molecular discovery. This analysis can, among other things, inform the design of improved substrate scoping studies; direct the prioritization of specific exemplar structures for synthesis; and substantiate claims of potential future applications for new methods.
Keywords: computational tools; lead-oriented synthesis; molecular discovery; molecular properties; synthetic chemistry.
© 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

