Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression
- PMID: 27573561
- PMCID: PMC5091322
- DOI: 10.1093/annonc/mdw402
Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression
Abstract
Background: The RAISE phase III clinical trial demonstrated that ramucirumab + FOLFIRI improved overall survival (OS) [hazard ratio (HR) = 0.844, P = 0.0219] and progression-free survival (PFS) (HR = 0.793, P < 0.0005) compared with placebo + FOLFIRI for second-line metastatic colorectal carcinoma (mCRC) patients previously treated with first-line bevacizumab, oxaliplatin, and a fluoropyrimidine. Since some patient or disease characteristics could be associated with differential efficacy or safety, prespecified subgroup analyses were undertaken. This report focuses on three of the most relevant ones: KRAS status (wild-type versus mutant), age (<65 versus ≥65 years), and time to progression (TTP) on first-line therapy (<6 versus ≥6 months).
Patients and methods: OS and PFS were evaluated by the Kaplan-Meier analysis, with HR determined by the Cox proportional hazards model. Treatment-by-subgroup interaction was tested to determine whether treatment effect was consistent between subgroup pairs.
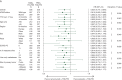
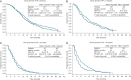
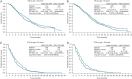
Results: Patients with both wild-type and mutant KRAS benefited from ramucirumab + FOLFIRI treatment over placebo + FOLFIRI (interaction P = 0.526); although numerically, wild-type KRAS patients benefited more (wild-type KRAS: median OS = 14.4 versus 11.9 months, HR = 0.82, P = 0.049; mutant KRAS: median OS = 12.7 versus 11.3 months, HR = 0.89, P = 0.263). Patients with both longer and shorter first-line TTP benefited from ramucirumab (interaction P = 0.9434), although TTP <6 months was associated with poorer OS (TTP ≥6 months: median OS = 14.3 versus 12.5 months, HR = 0.86, P = 0.061; TTP <6 months: median OS = 10.4 versus 8.0 months, HR = 0.86, P = 0.276). The subgroups of patients ≥65 versus <65 years also derived a similar ramucirumab survival benefit (interaction P = 0.9521) (≥65 years: median OS = 13.8 versus 11.7 months, HR = 0.85, P = 0.156; <65 years: median OS = 13.1 versus 11.9 months, HR = 0.86, P = 0.098). The safety profile of ramucirumab + FOLFIRI was similar across subgroups.
Conclusions: These analyses revealed similar efficacy and safety among patient subgroups with differing KRAS mutation status, longer or shorter first-line TTP, and age. Ramucirumab is a beneficial addition to second-line FOLFIRI treatment for a wide range of patients with mCRC.
Trial registration: ClinicalTrials.gov, NCT01183780.
Keywords: CRC; RAISE; VEGFR-2; metastatic colorectal carcinoma; phase III clinical trial; ramucirumab.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures


References
-
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(Suppl. 3): iii1–iii9. - PubMed
-
- Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: colon and rectum cancer. http://seer.cancer.gov/statfacts/html/colorect.html (22 February 2016, date last accessed).
-
- National Comprehensive Care Network. Clinical practice guidelines in oncology (NCCN guidelines®): colon cancer; rectal cancer. Version 2 2015. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf; http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (20 November 2015, date last accessed).
-
- Leung DW, Cachianes G, Kuang WJ et al. . Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246: 1306–1309. - PubMed
-
- Tugues S, Koch S, Gualandi L et al. . Vascular endothelial growth factors and receptors: anti-angiogenic therapy in the treatment of cancer. Mol Aspects Med 2011; 32: 88–111. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

