Clostridium difficile colitis: pathogenesis and host defence
- PMID: 27573580
- PMCID: PMC5109054
- DOI: 10.1038/nrmicro.2016.108
Clostridium difficile colitis: pathogenesis and host defence
Abstract
Clostridium difficile is a major cause of intestinal infection and diarrhoea in individuals following antibiotic treatment. Recent studies have begun to elucidate the mechanisms that induce spore formation and germination and have determined the roles of C. difficile toxins in disease pathogenesis. Exciting progress has also been made in defining the role of the microbiome, specific commensal bacterial species and host immunity in defence against infection with C. difficile. This Review will summarize the recent discoveries and developments in our understanding of C. difficile infection and pathogenesis.
Figures
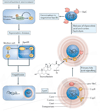


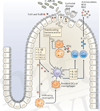
References
-
-
Lessa FC, et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015;372:825–834. This study provides a comprehensive assessment of the effect of C. difficile-associated disease on the current healthcare system in the United States.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

