Structural Diversity in Alkali Metal and Alkali Metal Magnesiate Chemistry of the Bulky 2,6-Diisopropyl-N-(trimethylsilyl)anilino Ligand
- PMID: 27573676
- PMCID: PMC5096043
- DOI: 10.1002/chem.201602683
Structural Diversity in Alkali Metal and Alkali Metal Magnesiate Chemistry of the Bulky 2,6-Diisopropyl-N-(trimethylsilyl)anilino Ligand
Abstract
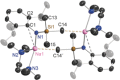
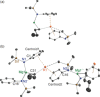
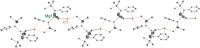
Bulky amido ligands are precious in s-block chemistry, since they can implant complementary strong basic and weak nucleophilic properties within compounds. Recent work has shown the pivotal importance of the base structure with enhancement of basicity and extraordinary regioselectivities possible for cyclic alkali metal magnesiates containing mixed n-butyl/amido ligand sets. This work advances alkali metal and alkali metal magnesiate chemistry of the bulky arylsilyl amido ligand [N(SiMe3 )(Dipp)]- (Dipp=2,6-iPr2 -C6 H3 ). Infinite chain structures of the parent sodium and potassium amides are disclosed, adding to the few known crystallographically characterised unsolvated s-block metal amides. Solvation by N,N,N',N'',N''-pentamethyldiethylenetriamine (PMDETA) or N,N,N',N'-tetramethylethylenediamine (TMEDA) gives molecular variants of the lithium and sodium amides; whereas for potassium, PMDETA gives a molecular structure, TMEDA affords a novel, hemi-solvated infinite chain. Crystal structures of the first magnesiate examples of this amide in [MMg{N(SiMe3 )(Dipp)}2 (μ-nBu)]∞ (M=Na or K) are also revealed, though these breakdown to their homometallic components in donor solvents as revealed through NMR and DOSY studies.
Keywords: alkali metals; amides; bases; magnesiates; metalation; structure elucidation.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures












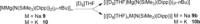
Similar articles
-
Heavy Alkali Metal Manganate Complexes: Synthesis, Structures and Solvent-Induced Dissociation Effects.Chemistry. 2022 Oct 4;28(55):e202201716. doi: 10.1002/chem.202201716. Epub 2022 Aug 3. Chemistry. 2022. PMID: 35775467 Free PMC article.
-
Lateral Metallation and Redistribution Reactions of Sodium Ferrates Containing Bulky 2,6-Diisopropyl-N-(trimethylsilyl)anilide Ligands.Chemistry. 2021 Nov 2;27(61):15180-15186. doi: 10.1002/chem.202102328. Epub 2021 Sep 12. Chemistry. 2021. PMID: 34324749 Free PMC article.
-
Developing a hetero-alkali-metal chemistry of 2,2,6,6-tetramethylpiperidide (TMP): stoichiometric and structural diversity within a series of lithium/sodium, lithium/potassium and sodium/potassium TMP compounds.Chemistry. 2011 Aug 1;17(32):8820-31. doi: 10.1002/chem.201101167. Epub 2011 Jul 15. Chemistry. 2011. PMID: 21766365 Free PMC article.
-
New Frontiers in Organosodium Chemistry as Sustainable Alternatives to Organolithium Reagents.Angew Chem Int Ed Engl. 2024 Jan 22;63(4):e202313556. doi: 10.1002/anie.202313556. Epub 2023 Oct 24. Angew Chem Int Ed Engl. 2024. PMID: 37801443 Review.
-
Structural Motifs of Alkali Metal Superbases in Non-coordinating Solvents.Chemistry. 2021 Jan 13;27(3):888-904. doi: 10.1002/chem.202002812. Epub 2020 Nov 9. Chemistry. 2021. PMID: 33165981 Free PMC article. Review.
Cited by
-
Heavy Alkali Metal Manganate Complexes: Synthesis, Structures and Solvent-Induced Dissociation Effects.Chemistry. 2022 Oct 4;28(55):e202201716. doi: 10.1002/chem.202201716. Epub 2022 Aug 3. Chemistry. 2022. PMID: 35775467 Free PMC article.
-
Bis(4-benzhydryl-benzoxazol-2-yl)methane - from a Bulky NacNac Alternative to a Trianion in Alkali Metal Complexes.Chemistry. 2021 Jul 7;27(38):9858-9865. doi: 10.1002/chem.202100616. Epub 2021 May 25. Chemistry. 2021. PMID: 34036637 Free PMC article.
-
Application of Bis(amido)alkyl Magnesiates toward the Synthesis of Molecular Rubidium and Cesium Hydrido-magnesiates.Organometallics. 2024 Jun 13;43(12):1393-1401. doi: 10.1021/acs.organomet.4c00190. eCollection 2024 Jun 24. Organometallics. 2024. PMID: 38938897 Free PMC article.
-
Lateral Metallation and Redistribution Reactions of Sodium Ferrates Containing Bulky 2,6-Diisopropyl-N-(trimethylsilyl)anilide Ligands.Chemistry. 2021 Nov 2;27(61):15180-15186. doi: 10.1002/chem.202102328. Epub 2021 Sep 12. Chemistry. 2021. PMID: 34324749 Free PMC article.
-
Regioselective functionalization of aryl azoles as powerful tool for the synthesis of pharmaceutically relevant targets.Nat Commun. 2020 Sep 7;11(1):4443. doi: 10.1038/s41467-020-18188-z. Nat Commun. 2020. PMID: 32895371 Free PMC article.
References
-
- Mulvey R. E., Robertson S. D., Angew. Chem. Int. Ed. 2013, 52, 11470–11487; - PubMed
- Angew. Chem. 2013, 125, 11682–11700.
-
- None
-
- Krasovskiy A., Krasovskaya V., Knochel P., Angew. Chem. Int. Ed. 2006, 45, 2958–2961; - PubMed
- Angew. Chem. 2006, 118, 3024–3027;
-
- García-Álvarez P., Graham D. V., Hevia E., Kennedy A. R., Klett J., Mulvey R. E., O'Hara C. T., Weatherstone S., Angew. Chem. Int. Ed. 2008, 47, 8079–8081; - PubMed
- Angew. Chem. 2008, 120, 8199–8201;
-
- Mosrin M., Knochel P., Org. Lett. 2008, 10, 2497–2500; - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

