Apoptosis repressor with caspase recruitment domain enhances survival and promotes osteogenic differentiation of human osteoblast cells under Zoledronate treatment
- PMID: 27573706
- PMCID: PMC5042767
- DOI: 10.3892/mmr.2016.5669
Apoptosis repressor with caspase recruitment domain enhances survival and promotes osteogenic differentiation of human osteoblast cells under Zoledronate treatment
Abstract
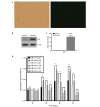
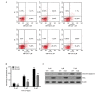
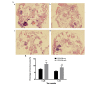
Zoledronate is one of the most potent nitrogen-containing bisphosphonates which has been demonstrated to result in osteoblast apoptosis and impact osteogenic differentiation in vitro. This effect of Zoledronate on osteoblasts may partially explain bisphosphonate‑associated osteonecrosis of the jaw, a serious complication associated with treatment with bisphosphonates. Apoptosis repressor with caspase recruitment domain (ARC) is a multifunctional inhibitor of apoptosis that is physiologically expressed predominantly in post‑mitotic cells such as cardiomyocytes, neurons and skeletal muscle cells. However, its effect on human osteoblasts remains unclear. The current study aimed to investigate the effects of ARC on human osteoblasts under the treatment of high concentrations of Zoledronate. ARC‑overexpressed human osteoblasts were established and were exposed to Zoledronate with different concentrations (0, 1 and 5 µM) in vitro. Cell numbers were detected using the MTT assay, and flow cytometry was used to identity cell apoptosis. Alkaline phosphatase staining, quantitative analysis and ectopic osteogenesis in nude mice were used to evaluate the osteogenic differentiation of ARC‑overexpressed osteoblasts. It was observed that ARC is able to reverse the inhibitory effect of Zoldronate on osteoblasts. ARC is additionally able to promote osteogenic differentiation of osteoblasts and inhibit their apoptosis. These observations suggest a critical role for ARC in the regulation of human osteoblasts under Zoledronate treatment.
Figures

Similar articles
-
Inhibition of osteoblast function in vitro by aminobisphosphonates.J Cell Biochem. 2009 Jan 1;106(1):109-18. doi: 10.1002/jcb.21983. J Cell Biochem. 2009. PMID: 19003973
-
Dose-dependent metabolic effect of zoledronate on primary human osteoblastic cell cultures.Clin Exp Rheumatol. 2010 Nov-Dec;28(6):873-9. Epub 2011 Jan 3. Clin Exp Rheumatol. 2010. PMID: 21205463
-
Zoledronate, ibandronate and clodronate enhance osteoblast differentiation in a dose dependent manner--a quantitative in vitro gene expression analysis of Dlx5, Runx2, OCN, MSX1 and MSX2.J Craniomaxillofac Surg. 2011 Dec;39(8):562-9. doi: 10.1016/j.jcms.2010.10.007. Epub 2010 Oct 28. J Craniomaxillofac Surg. 2011. PMID: 21030265
-
Bisphosphonates: effects on osteoblast.Eur J Clin Pharmacol. 2012 Jul;68(7):1013-8. doi: 10.1007/s00228-012-1216-7. Epub 2012 Feb 9. Eur J Clin Pharmacol. 2012. PMID: 22318756 Review.
-
Apoptosis repressor with caspase recruitment domain, a multifunctional modulator of cell death.J Cell Mol Med. 2011 May;15(5):1044-53. doi: 10.1111/j.1582-4934.2010.01221.x. J Cell Mol Med. 2011. PMID: 21129150 Free PMC article. Review.
Cited by
-
Role of apoptosis repressor with caspase recruitment domain in human health and chronic diseases.Ann Med. 2024 Dec;56(1):2409958. doi: 10.1080/07853890.2024.2409958. Epub 2024 Oct 1. Ann Med. 2024. PMID: 39351758 Free PMC article. Review.
-
Apoptosis repressor with caspase recruitment domain (ARC) promotes bone regeneration of bone marrow-derived mesenchymal stem cells by activating Fgf-2/PI3K/Akt signaling.Stem Cell Res Ther. 2021 Mar 16;12(1):185. doi: 10.1186/s13287-021-02253-5. Stem Cell Res Ther. 2021. PMID: 33726822 Free PMC article.
-
Transcriptomic Profile of Human Osteoblast-like Cells Grown on Trabecular Titanium.Int J Mol Sci. 2025 Apr 11;26(8):3598. doi: 10.3390/ijms26083598. Int J Mol Sci. 2025. PMID: 40332083 Free PMC article.
-
17β-estradiol promotes osteogenic differentiation of BMSCs by regulating mitophagy through ARC.J Orthop Surg Res. 2025 Jan 10;20(1):35. doi: 10.1186/s13018-024-05400-9. J Orthop Surg Res. 2025. PMID: 39794817 Free PMC article.
-
Role of apoptosis repressor with caspase recruitment domain (ARC) in cancer.Oncol Lett. 2019 Dec;18(6):5691-5698. doi: 10.3892/ol.2019.10981. Epub 2019 Oct 11. Oncol Lett. 2019. PMID: 31788041 Free PMC article. Review.
References
-
- Tenta R, Sourla A, Lembessis P, Koutsilieris M. Bone-related growth factors and zoledronic acid regulate the PTHrP/PTH.1 receptor bioregulation systems in MG-63 human osteosarcoma cells. Anticancer Res. 2006;26:283–291. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

