Dynamics of sensorimotor cortex activation during absence and myoclonic seizures in a mouse model of juvenile myoclonic epilepsy
- PMID: 27573707
- PMCID: PMC5056152
- DOI: 10.1111/epi.13493
Dynamics of sensorimotor cortex activation during absence and myoclonic seizures in a mouse model of juvenile myoclonic epilepsy
Abstract
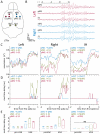
Objective: Generalized epilepsy syndromes often confer multiple types of seizures, but it is not known if these seizures activate separate or overlapping brain networks. Recently, we reported that mice with a juvenile myoclonic epilepsy mutation (Gabra1[A322D]) exhibited both absence and myoclonic generalized seizures. Here, we determined the time course of sensorimotor cortex activation and the spatial distribution of spike voltage during these two seizures.
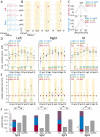
Methods: We implanted Gabra1+/A322D mice with multiple electroencephalography (EEG) electrodes over bilateral somatosensory cortex barrel fields (S1) and anterior (aM1) and posterior (pM1) motor cortices and recorded absence seizures/spike-wave discharges (SWDs) and myoclonic seizures. We used nonlinear-association analyses and cross-correlation calculations to determine the strength, leading regions, and time delays of cortical coupling from the preictal to ictal states and within the spike and interspike periods. The distribution of spike voltage was also measured in SWDs and myoclonic seizures.
Results: EEG connectivity among all electrode pairs increased at the onset of both SWDs and myoclonic seizures. Surprisingly, during spikes of both seizure types, S1 led M1 with similar delay times. Myoclonic seizure spikes started more focally than SWD spikes, with a significant majority appearing first only in S1 electrodes, whereas a substantial fraction of SWD spikes were detected first in S1 and at least one M1 electrode. The absolute voltage of myoclonic seizure spikes was significantly higher than that of SWD spikes, and there was a greater relative voltage over M1 during myoclonic seizure spikes than in the first one to two SWD spikes.
Significance: The leading sites in S1 and similar delay times suggest both SWDs and myoclonic seizures activate overlapping networks in sensorimotor cortex and thus, therapeutically targeting of this network could potentially treat both seizures. Spike focality, absolute voltage, and voltage distribution provide insight into neuronal activation during these two seizure types.
Keywords: Absence seizure; Electroencephalography; GABAA receptor; Generalized seizure; Network analysis.
Wiley Periodicals, Inc. © 2016 International League Against Epilepsy.
Figures


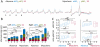

Similar articles
-
Cortical activation in generalized seizures.Epilepsia. 2019 Sep;60(9):1932-1941. doi: 10.1111/epi.16306. Epub 2019 Jul 31. Epilepsia. 2019. PMID: 31368118 Free PMC article.
-
The developmental evolution of the seizure phenotype and cortical inhibition in mouse models of juvenile myoclonic epilepsy.Neurobiol Dis. 2015 Oct;82:164-175. doi: 10.1016/j.nbd.2015.05.016. Epub 2015 Jun 6. Neurobiol Dis. 2015. PMID: 26054439 Free PMC article.
-
Changes in corticocortical and corticohippocampal network during absence seizures in WAG/Rij rats revealed with time varying Granger causality.Epilepsy Behav. 2016 Nov;64(Pt A):44-50. doi: 10.1016/j.yebeh.2016.08.009. Epub 2016 Oct 11. Epilepsy Behav. 2016. PMID: 27728902
-
Neurophysiology of juvenile myoclonic epilepsy.Epilepsy Behav. 2013 Jul;28 Suppl 1:S30-9. doi: 10.1016/j.yebeh.2012.11.042. Epilepsy Behav. 2013. PMID: 23756477 Review.
-
Transition issues for benign epilepsy with centrotemporal spikes, nonlesional focal epilepsy in otherwise normal children, childhood absence epilepsy, and juvenile myoclonic epilepsy.Epilepsia. 2014 Aug;55 Suppl 3:16-20. doi: 10.1111/epi.12706. Epilepsia. 2014. PMID: 25209080 Review.
Cited by
-
Preoptic area influences sleep-related seizures in a genetic epilepsy mouse model.Cereb Cortex. 2025 Jul 1;35(7):bhaf187. doi: 10.1093/cercor/bhaf187. Cereb Cortex. 2025. PMID: 40693751 Free PMC article.
-
The impact of early-life environment on absence epilepsy and neuropsychiatric comorbidities.IBRO Neurosci Rep. 2022 Nov 9;13:436-468. doi: 10.1016/j.ibneur.2022.10.012. eCollection 2022 Dec. IBRO Neurosci Rep. 2022. PMID: 36386598 Free PMC article. Review.
-
Preoptic area controls sleep-related seizure onset in a genetic epilepsy mouse model.bioRxiv [Preprint]. 2024 Sep 14:2023.11.24.568593. doi: 10.1101/2023.11.24.568593. bioRxiv. 2024. Update in: Cereb Cortex. 2025 Jul 1;35(7):bhaf187. doi: 10.1093/cercor/bhaf187. PMID: 39314442 Free PMC article. Updated. Preprint.
-
Sleep slow-wave oscillations trigger seizures in a genetic epilepsy model of Dravet syndrome.Brain Commun. 2022 Dec 17;5(1):fcac332. doi: 10.1093/braincomms/fcac332. eCollection 2023. Brain Commun. 2022. PMID: 36632186 Free PMC article.
-
Neuronal rhythmicity and cortical arousal in a mouse model of absence epilepsy.Exp Neurol. 2024 Nov;381:114925. doi: 10.1016/j.expneurol.2024.114925. Epub 2024 Aug 14. Exp Neurol. 2024. PMID: 39151596 Free PMC article.
References
-
- Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676–85. - PubMed
-
- Genton P, Thomas P, Kasteleijn-Nolst Trenite DG, Medina MT, Salas-Puig J. Clinical aspects of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S8–14. - PubMed
-
- Martinez-Juarez IE, Alonso ME, Medina MT, et al. Juvenile myoclonic epilepsy subsyndromes: family studies and long-term follow-up. Brain. 2006;129:1269–80. - PubMed
-
- Senf P, Schmitz B, Holtkamp M, Janz D. Prognosis of juvenile myoclonic epilepsy 45 years after onset: Seizure outcome and predictors. Neurology. 2013;81:2128–33. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

