Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis
- PMID: 27573815
- PMCID: PMC5030797
- DOI: 10.1084/jem.20151613
Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis
Abstract
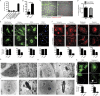
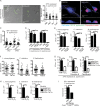
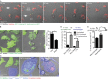
Inflammasomes activate caspase-1 in response to cytosolic contamination or perturbation. This inflammatory caspase triggers the opening of the GSDMD pore in the plasma membrane, resulting in lytic cell death called pyroptosis. We had previously assumed that pyroptosis releases intracellular bacteria to the extracellular space. Here, we find that viable bacteria instead remain trapped within the cellular debris of pyroptotic macrophages. This trapping appears to be an inevitable consequence of how osmotic lysis ruptures the plasma membrane, and may also apply to necroptosis and some forms of nonprogrammed necrosis. Although membrane tears release soluble cytosolic contents, they are small enough to retain organelles and bacteria. We call this structure the pore-induced intracellular trap (PIT), which is conceptually parallel to the neutrophil extracellular trap (NET). The PIT coordinates innate immune responses via complement and scavenger receptors to drive recruitment of and efferocytosis by neutrophils. Ultimately, this secondary phagocyte kills the bacteria. Hence, caspase-1-driven pore-induced cell death triggers a multifaceted defense against intracellular bacteria facilitated by trapping the pathogen within the cellular debris. Bona fide intracellular bacterial pathogens, such as Salmonella, must prevent or delay pyroptosis to avoid being trapped in the PIT and subsequently killed by neutrophils.
© 2016 Jorgensen et al.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

