p62 improves AD-like pathology by increasing autophagy
- PMID: 27573878
- PMCID: PMC5479312
- DOI: 10.1038/mp.2016.139
p62 improves AD-like pathology by increasing autophagy
Retraction in
-
Retraction Note: p62 improves AD-like pathology by increasing autophagy.Mol Psychiatry. 2021 Jul;26(7):3664. doi: 10.1038/s41380-020-0854-x. Mol Psychiatry. 2021. PMID: 32782379 Free PMC article. No abstract available.
Abstract
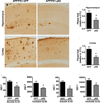
The multifunctional protein p62 is associated with neuropathological inclusions in several neurodegenerative disorders, including frontotemporal lobar degeneration, amyotrophic lateral sclerosis and Alzheimer's disease (AD). Strong evidence shows that in AD, p62 immunoreactivity is associated with neurofibrillary tangles and is involved in tau degradation. However, it remains to be determined whether p62 also plays a role in regulating amyloid-β (Aβ) aggregation and degradation. Using a gene therapy approach, here we show that increasing brain p62 expression rescues cognitive deficits in APP/PS1 mice, a widely used animal model of AD. The cognitive improvement was associated with a decrease in Aβ levels and plaque load. Using complementary genetic and pharmacologic approaches, we found that the p62-mediated changes in Aβ were due to an increase in autophagy. To this end, we showed that removing the LC3-interacting region of p62, which facilitates p62-mediated selective autophagy, or blocking autophagy with a pharmacological inhibitor, was sufficient to prevent the decrease in Aβ. Overall, we believe these data provide the first direct in vivo evidence showing that p62 regulates Aβ turnover.
Conflict of interest statement
The authors declare no conflict of interest
Figures




References
-
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19(8):983–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

