Dual regulation of the native ClC-K2 chloride channel in the distal nephron by voltage and pH
- PMID: 27574292
- PMCID: PMC5004338
- DOI: 10.1085/jgp.201611623
Dual regulation of the native ClC-K2 chloride channel in the distal nephron by voltage and pH
Abstract
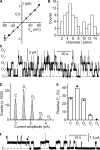
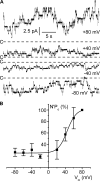
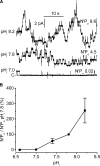
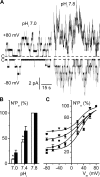
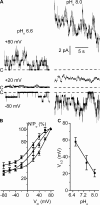
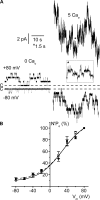
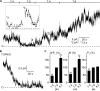
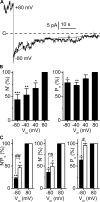
ClC-K2, a member of the ClC family of Cl(-) channels and transporters, forms the major basolateral Cl(-) conductance in distal nephron epithelial cells and therefore plays a central role in renal Cl(-) absorption. However, its regulation remains largely unknown because of the fact that recombinant ClC-K2 has not yet been studied at the single-channel level. In the present study, we investigate the effects of voltage, pH, Cl(-), and Ca(2+) on native ClC-K2 in the basolateral membrane of intercalated cells from the mouse connecting tubule. The ∼10-pS channel shows a steep voltage dependence such that channel activity increases with membrane depolarization. Intracellular pH (pHi) and extracellular pH (pHo) differentially modulate the voltage dependence curve: alkaline pHi flattens the curve by causing an increase in activity at negative voltages, whereas alkaline pHo shifts the curve toward negative voltages. In addition, pHi, pHo, and extracellular Ca(2+) strongly increase activity, mainly because of an increase in the number of active channels with a comparatively minor effect on channel open probability. Furthermore, voltage alters both the number of active channels and their open probability, whereas intracellular Cl(-) has little influence. We propose that changes in the number of active channels correspond to them entering or leaving an inactivated state, whereas modulation of open probability corresponds to common gating by these channels. We suggest that pH, through the combined effects of pHi and pHo on ClC-K2, might be a key regulator of NaCl absorption and Cl(-)/HCO3 (-) exchange in type B intercalated cells.
© 2016 Pinelli et al.
Figures
Similar articles
-
Dietary K+ and Cl- independently regulate basolateral conductance in principal and intercalated cells of the collecting duct.Pflugers Arch. 2018 Feb;470(2):339-353. doi: 10.1007/s00424-017-2084-x. Epub 2017 Nov 13. Pflugers Arch. 2018. PMID: 29134279 Free PMC article.
-
Adenosine inhibits the basolateral Cl- ClC-K2/b channel in collecting duct intercalated cells.Am J Physiol Renal Physiol. 2020 Apr 1;318(4):F870-F877. doi: 10.1152/ajprenal.00572.2019. Epub 2020 Jan 27. Am J Physiol Renal Physiol. 2020. PMID: 31984792 Free PMC article.
-
The ClC-K2 Chloride Channel Is Critical for Salt Handling in the Distal Nephron.J Am Soc Nephrol. 2017 Jan;28(1):209-217. doi: 10.1681/ASN.2016010085. Epub 2016 Jun 22. J Am Soc Nephrol. 2017. PMID: 27335120 Free PMC article.
-
Exploration of the basolateral chloride channels in the renal tubule using.Nephron Physiol. 2005;99(2):p64-8. doi: 10.1159/000082972. Epub 2004 Dec 28. Nephron Physiol. 2005. PMID: 15627805 Review.
-
Expression and function of CLC and cystic fibrosis transmembrane conductance regulator chloride channels in renal epithelial tubule cells: pathophysiological implications.Chang Gung Med J. 2007 Jan-Feb;30(1):17-25. Chang Gung Med J. 2007. PMID: 17477025 Review.
Cited by
-
α-Ketoglutarate stimulates pendrin-dependent Cl- absorption in the mouse CCD through protein kinase C.Am J Physiol Renal Physiol. 2018 Jul 1;315(1):F7-F15. doi: 10.1152/ajprenal.00576.2017. Epub 2018 Feb 7. Am J Physiol Renal Physiol. 2018. PMID: 29412702 Free PMC article.
-
Reduced Membrane Insertion of CLC-K by V33L Barttin Results in Loss of Hearing, but Leaves Kidney Function Intact.Front Physiol. 2017 May 15;8:269. doi: 10.3389/fphys.2017.00269. eCollection 2017. Front Physiol. 2017. PMID: 28555110 Free PMC article.
-
Angiotensin II increases activity of the ClC-K2 Cl- channel in collecting duct intercalated cells by stimulating production of reactive oxygen species.J Biol Chem. 2021 Jan-Jun;296:100347. doi: 10.1016/j.jbc.2021.100347. Epub 2021 Jan 30. J Biol Chem. 2021. PMID: 33524393 Free PMC article.
-
The Renal Physiology of Pendrin-Positive Intercalated Cells.Physiol Rev. 2020 Jul 1;100(3):1119-1147. doi: 10.1152/physrev.00011.2019. Physiol Rev. 2020. PMID: 32347156 Free PMC article. Review.
-
DHHC7-mediated palmitoylation of the accessory protein barttin critically regulates the functions of ClC-K chloride channels.J Biol Chem. 2020 May 1;295(18):5970-5983. doi: 10.1074/jbc.RA119.011049. Epub 2020 Mar 17. J Biol Chem. 2020. PMID: 32184353 Free PMC article.
References
-
- Andrini O., Keck M., L’Hoste S., Briones R., Mansour-Hendili L., Grand T., Sepúlveda F.V., Blanchard A., Lourdel S., Vargas-Poussou R., and Teulon J.. 2014. CLCNKB mutations causing mild Bartter syndrome profoundly alter the pH and Ca2+ dependence of ClC-Kb channels. Pflugers Arch. 466:1713–1723. 10.1007/s00424-013-1401-2 - DOI - PubMed
-
- Chambrey R., Kurth I., Peti-Peterdi J., Houillier P., Purkerson J.M., Leviel F., Hentschke M., Zdebik A.A., Schwartz G.J., Hübner C.A., and Eladari D.. 2013. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc. Natl. Acad. Sci. USA. 110:7928–7933. 10.1073/pnas.1221496110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

