Neural-metabolic coupling in the central visual pathway
- PMID: 27574310
- PMCID: PMC5003858
- DOI: 10.1098/rstb.2015.0357
Neural-metabolic coupling in the central visual pathway
Abstract
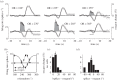
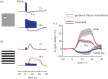
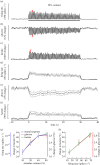
Studies are described which are intended to improve our understanding of the primary measurements made in non-invasive neural imaging. The blood oxygenation level-dependent signal used in functional magnetic resonance imaging (fMRI) reflects changes in deoxygenated haemoglobin. Tissue oxygen concentration, along with blood flow, changes during neural activation. Therefore, measurements of tissue oxygen together with the use of a neural sensor can provide direct estimates of neural-metabolic interactions. We have used this relationship in a series of studies in which a neural microelectrode is combined with an oxygen micro-sensor to make simultaneous co-localized measurements in the central visual pathway. Oxygen responses are typically biphasic with small initial dips followed by large secondary peaks during neural activation. By the use of established visual response characteristics, we have determined that the oxygen initial dip provides a better estimate of local neural function than the positive peak. This contrasts sharply with fMRI for which the initial dip is unreliable. To extend these studies, we have examined the relationship between the primary metabolic agents, glucose and lactate, and associated neural activity. For this work, we also use a Doppler technique to measure cerebral blood flow (CBF) together with neural activity. Results show consistent synchronously timed changes such that increases in neural activity are accompanied by decreases in glucose and simultaneous increases in lactate. Measurements of CBF show clear delays with respect to neural response. This is consistent with a slight delay in blood flow with respect to oxygen delivery during neural activation.This article is part of the themed issue 'Interpreting BOLD: a dialogue between cognitive and cellular neuroscience'.
Keywords: blood flow; glucose; lactate; neural activity; tissue oxygen; visual system.
© 2016 The Author(s).
Figures





Similar articles
-
Neurometabolic coupling between neural activity, glucose, and lactate in activated visual cortex.J Neurochem. 2015 Nov;135(4):742-54. doi: 10.1111/jnc.13143. Epub 2015 May 29. J Neurochem. 2015. PMID: 25930947 Free PMC article.
-
Validation and optimization of hypercapnic-calibrated fMRI from oxygen-sensitive two-photon microscopy.Philos Trans R Soc Lond B Biol Sci. 2016 Oct 5;371(1705):20150359. doi: 10.1098/rstb.2015.0359. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27574311 Free PMC article.
-
High-resolution neurometabolic coupling revealed by focal activation of visual neurons.Nat Neurosci. 2004 Sep;7(9):919-20. doi: 10.1038/nn1308. Epub 2004 Aug 22. Nat Neurosci. 2004. PMID: 15322552
-
Submillimeter-resolution fMRI: Toward understanding local neural processing.Prog Brain Res. 2016;225:123-52. doi: 10.1016/bs.pbr.2016.03.003. Epub 2016 Apr 1. Prog Brain Res. 2016. PMID: 27130414 Review.
-
The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal.Philos Trans R Soc Lond B Biol Sci. 2002 Aug 29;357(1424):1003-37. doi: 10.1098/rstb.2002.1114. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12217171 Free PMC article. Review.
Cited by
-
Traces of EEG-fMRI coupling reveals neurovascular dynamics on sleep inertia.Sci Rep. 2024 Jan 17;14(1):1537. doi: 10.1038/s41598-024-51694-4. Sci Rep. 2024. PMID: 38233587 Free PMC article.
-
Increased Retinal Metabolism Induced by Flicker in the Isolated Mouse Retina.eNeuro. 2024 May 10;11(5):ENEURO.0509-23.2024. doi: 10.1523/ENEURO.0509-23.2024. Print 2024 May. eNeuro. 2024. PMID: 38641415 Free PMC article.
-
The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease.Neuron. 2017 Sep 27;96(1):17-42. doi: 10.1016/j.neuron.2017.07.030. Neuron. 2017. PMID: 28957666 Free PMC article. Review.
-
Brain Tissue Oxygen and BOLD fMRI Under Different Levels of Neuronal Activity.Adv Exp Med Biol. 2023;1438:3-8. doi: 10.1007/978-3-031-42003-0_1. Adv Exp Med Biol. 2023. PMID: 37845431 Free PMC article.
-
An fMRI Investigation into the Effects of Ketogenic Medium-Chain Triglycerides on Cognitive Function in Elderly Adults: A Pilot Study.Nutrients. 2021 Jun 22;13(7):2134. doi: 10.3390/nu13072134. Nutrients. 2021. PMID: 34206642 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

